![]()
Razzaque MS (ed): Phosphate and Vitamin D in Chronic Kidney Disease.
Contrib Nephrol. Basel, Karger, 2013, vol 180, pp 25-46 (DOI: 10.1159/000346777)
______________________
Complex Regulation and Diverse Functions of Alpha-Klotho
Ryota Maeda · Akihiro Imura · Yo-ichi Nabeshima
Laboratory of Molecular Life Science, Foundation for Biomedical Research and Innovation, Kobe, Japan
______________________
Abstract
It has been understood that the α-klotho gene, first identified as an aging-related gene, is actually necessary for regulating mineral homeostasis in vertebrates. All vertebrates, including humans, actively maintain calcium and phosphate ions in bones, circulating blood, and cerebrospinal fluid. Therefore, disruptions in homeostasis cause osteoporosis, ectopic calcification, and epilepsy. Vitamin D and parathyroid hormone are well-known hormones for maintaining calcium and phosphate balance. Synthesis of vitamin D, secretion of parathyroid hormone, and absorption of calcium and phosphate ions are all finely controlled by α-Klotho-dependent machineries. However, the precise molecular mechanisms and functions of α-Klotho are still unclear. In this article, we present an overview of recent progress in α-klotho research, with a main focus on molecular aspects.
Copyright © 2013 S. Karger AG, Basel
In whole body fluids, concentrations of mineral ions, e.g. sodium ions, potassium ions, chloride ions, protons, hydrogen carbonate ions, heavy metal ions, calcium ions and phosphate ions, are tightly regulated within a certain ranges. If the concentrations of these ions decrease or increase in body fluids, several disorders could occur including hypertension, infarction, acidosis and calcification. Osteoporosis, skin atrophy, or arterial sclerosis is often observed especially in aged humans and is caused by their decreased ability to maintain calcium ion and phosphate ion homeostasis. Concentrations of calcium and phosphate ions are partially controlled via hormones, vitamin D and parathyroid hormone (PTH). Historically and clinically, it has been well known that these hormones are necessary for maintaining a calcium and phosphate ion balance within physiological concentrations. Furthermore, recent work has clarified that α-klotho, which was discovered as an aging-related gene, is also necessary for calcium and phosphate homeostasis.
In the aging process, irreversible damage to proteins and genomes accumulate in the whole body due to oxidative stress, ultraviolet damage, and chemicals. Consequently, any kind of homeostasis would not be able to be finely maintained. It can be said that the capability of maintaining homeostasis is directly connected to the aging process [1-7]. Therefore, α-klotho-deficient mice can be used as an aging model in mice, especially in mineral homeostatic disorders [8, 9]. Disorders such as osteoporosis, skin atrophy, and arterial sclerosis are all seen in α-klotho-deficient mice. Recent work showed that excessive vitamin D synthesis causes these aging-related phenotypes. Therefore, α-klotho can be considered more precisely as a gene which regulates mineral homeostasis [10, 11].
Evolution
A freshwater fish of teleosts, zebrafish (Danio rerio), has an α-klotho homologous gene [12]. The transcript is exclusively expressed in the gill, which is the origin of the parathyroid gland and where mineral ions are actively exchanged [13]. It has been reported that a putative homologous gene of mammalian α-klotho genes is expressed in the fruit fly (Drosophila) and nematode (Caenorhabditis elegans) [14, 15]. However, proposed α-klotho homologue genes are distinguished from mammalian α-klotho by a few points: the two genes (1) code cytosolic protein without signal peptides or transmembrane domains, (2) have only a KL1-like domain, and (3) conserves two catalytic glutamate residues. Therefore, it is not plausible that α-klotho homologous genes proposed in the fruit fly and nematode are indeed functional homologues of mammalian α-klotho genes. In these points, the two proposed genes are rather similar to the mammalian Klotho-related protein (KLrP) gene.
Molecular Aspects
The klotho gene, now renamed as the α-klotho gene (see below), was first described in 1997. The phenotype of mice deficient in the gene resembled that of aged humans [16]. The reported original mice were obtained as an α-klotho gene hypomorph strain, in which expression levels of the transcript were reduced due to an insertion mutation upstream of the gene. Then, α-klotho complete null mice were generated in 2003 [17]. The overall phenotype of α-klotho knockout mice was similar to that of α-klotho hypomorph mice except that in α-klotho hypomorph mice, the protein expression of α-Klotho will be induced under some circumstances [18].
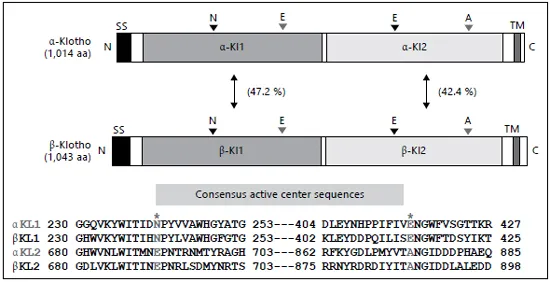
Fig. 1. Schematic diagrams of structural similarities of α-klotho and β-klotho. The acid/ base and nucleophilic amino acids are indicated.
The transcript of the α-klotho gene encodes a single-pass type-I transmembrane protein with two external glycosidase-like domains. The translated protein is modified, most likely by N-glycosylation, and is observed as at least two molecular masses between 120 and 135 kDa [19]. A short splicing variant has been detected, but any translated proteins of the short transcript have not been observed at this time [19-21].
It is quite an interesting feature that two glutamate residues, which are indispensable for glycosidase activity, are not conserved in the two glycosidase-like domains of α-klotho [22, 23]. In the N-terminal KL1 domain, the acid/base glutamate is changed to asparagine and, in the C-terminal KL2 domain, nucleophilic glutamate is replaced by alanine or serine. In 2004, another high homologous gene of α-klotho was identified and named the β-klotho gene, which also encodes a single-pass type-I transmembrane protein with two external glycosidase-like domains. Catalytic glutamate residues are replaced in the same way as above [24, 25]. Therefore, we have referred to the original klotho gene as the α-klotho gene according to natural nomenclature (fig. 1).
Structures
α-Klotho was classified into a subgroup of glycoside hydrolase (GH) family 1 with β-Klotho, LPH (lactase-phlorizin hydrolase), KLrP, and CBG (cytosolic β-glucosidase) [23]. A couple of crystal structures of mammalian β-glycosidases of the GH family 1 have been solved [26]. Both the structures of KLrP and CBG consist of a (β/α) 8 TIM barrel of β-strands, in which the acid/base catalyst is located on strand 4 and nucleophilic glutamate is on strand 7. The cavity of the active center was occupied with lipids or steroids. Based on structural studies of GH family 1, it is possible that α-Klotho also recognizes some small molecules, such as sugars and lipids [26].
KLrP (klotho-related protein) is the closest homologue of α-Klotho and β-Klotho proteins (fig. 1). The crystal of KLrP was formed with a glucose-ceramide, which was docked at its active center. Moreover, two lipids were also contained in the crystal. The mutated KLrP that the acid/base glutamate was replaced to glutamine with was crystallized with glucose. Glucose was bound at the active center in the crystal as well [26]. Any crystal structures of α-Klotho or β-Klotho have not yet been solved. It is expected that determination of the structure will provide information about how α-Klotho specifically recognizes glucuronide and steroids.
Expression and Distribution
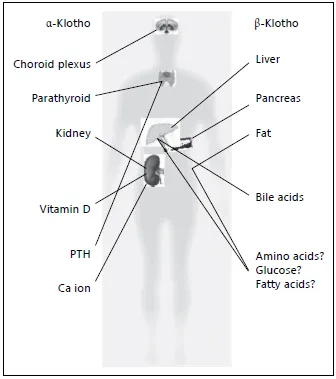
In the original report of the murine α-klotho gene, the expression pattern of the transcripts of the gene was analyzed via Northern blotting, RT-PCR, and in situ hybridization. The highest expression was detected in the distal convoluted tubule (DCT) of the kidney and in the choroid plexus of the brain [16].
Protein expression in mice was confirmed with anti-α-Klotho antibodies [19]. At least two types of α-Klotho protein were found, namely the membrane-bound type and the secreted one. The expression of membranebound α-Klotho is restricted in the DCT of the kidney, in the choroid plexus of the brain where mineral ions are actively transported and, of particular interest, in the parathyroid glands where PTH is synthesized to be secreted (fig. 2). Recently, α-Klotho expression was confirmed in syncytiotrophoblasts in normal term placenta, where nutrients are exchanged between mother and fetus and where progesterone is secreted [27]. Therefore, it is noticeable that α-Klotho is expressed in tissues where mineral ions are actively regulated and where hormones are secreted. On the other hand, secreted α-Klotho was detected not only in plasma, but also in cerebrospinal fluid (CSF) and urine [19].
Fig. 2. A Schematic diagram summarizing expressions and functions of α-klotho and β-klotho.
We investigated the subcellular distribution of the α-Klotho protein using extracted choroid plexus in mice. In ex vivo studies, almost all α-Klotho protein was localized in intracellular compartments, but the amount of α-Klotho on the tissue surface was slight. Low extracellular calcium concentrations (~0.8 m M) induced the secretion of α-Klotho from the choroid plexus, the parathyroid glands, and the kidney [28, 29]. Secretion was reconstruc...