![]()
Section II: Topical Treatment of Impaired Mucosal Membranes
Surber C, Elsner P, Farage MA (eds): Topical Applications and the Mucosa.
Curr Probl Dermatol. Basel, Karger, 2011, vol 40, pp 20–35
____________________________
Nasal Drug Delivery in Humans
Christoph Bitter a • Katja Suter-Zimmermanna • Christian Surbera,b
aHospital Pharmacy and bDepartment of Dermatology, University of Basel Hospital, Basel, Switzerland
____________________________
Abstract
Intranasal administration is an attractive option for local and systemic delivery of many therapeutic agents. The nasal mucosa is - compared to other mucosae - easily accessible. Intranasal drug administration is noninvasive, essentially painless and particularly suited for children. Application can be performed easily by patients or by physicians in emergency settings. Intranasal drug delivery offers a rapid onset of therapeutic effects (local or systemic). Nasal application circumvents gastrointestinal degradation and hepatic first-pass metabolism of the drug. The drug, the vehicle and the application device form an undividable triad. Its selection is therefore essential for the successful development of effective nasal products. This paper discusses the feasibility and potential of intranasal administration. A series of questions regarding (a) the intended use (therapeutic considerations), (b) the drug, (c) the vehicle and (d) the application device (pharmaceutical considerations) are addressed with a view to their impact on the development of products for nasal application. Current and future trends and perspectives are discussed.
Copyright © 2011 S. Karger AG, Basel
Intranasal administration offers a variety of attractive options for local and systemic delivery of diverse therapeutic agents. The nature of the nasal mucosa provides a series of unique attributes, all of which may help to maximize the patient’s safety, convenience and compliance.
The nasal mucosa is - compared to other mucous membranes - easily accessible and provides a practical entrance portal for small and large molecules. Intranasal administration offers a rapid onset of therapeutic effects, no first-pass effect, no gastrointestinal degradation or lung toxicity, noninvasiveness, essentially painless application, and easy and ready use by patients - particularly suited for children - or by physicians in emergency settings. More recently a nasal influenza vaccine spray (Flu Mist®) has been successfully introduced. The chances for direct nose-to-brain drug delivery are currently the subject of controversial debates [1, 2].
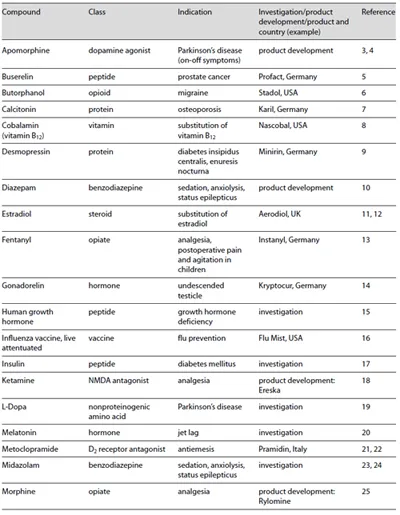
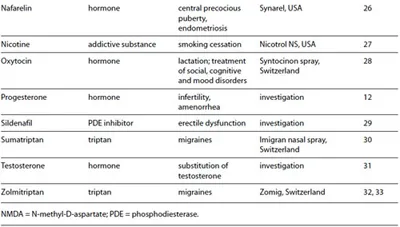
Given these positive attributes, it is obvious to consider intranasal administration when improving the profile of existing drugs including life cycle management or when developing new therapeutics. A quick glance at the market and at current research activities confirms the attractiveness of intranasal drug administration. Table 1 shows selected drugs for intranasal administration with systemic effects.
In order to estimate the feasibility and potential of intranasal administration, a series of questions regarding (a) the intended use (therapeutic considerations), (b) the drug, (c) the vehicle and (d) the application device (pharmaceutical considerations) have to be addressed, e.g.:
Table 1. Selection of compounds for transmucosal nasal drug delivery
(a) Is the drug designated for local or systemic delivery, for single or repetitive administration, is the therapeutic target concentration known?
(b) Are the physicochemical properties of the drug suitable for intranasal administration, can clinically relevant bioavailability be achieved?
(c) Can the vehicle provide prolonged drug stability, ideal characteristics during (ejection) and after application (prolonged residence time on the mucosa) and support drug delivery to local target tissues or to the blood vessels for systemic delivery?
(d) And finally, is the application device easily deployable and does it allow adequate drug/formulation deposition within the nose?
These issues are addressed below with a view to their impact on the development of products for nasal application.
The Nose - Anatomy and Function
The nose is a complex multifunctional organ. The major functions of the nasal cavity comprise cleansing the inhaled air and olfaction. Moreover, it exerts important protective and supportive activities; it filters, heats and humidifies the inhaled air before it reaches the lower parts of the airways. Nasal hairs and mainly the nasal mucosa with its sticky mucus blanket help to prevent xenobiotics like allergens, pathogens or foreign particles from reaching the lungs. It represents a most efficient first line of defense for the body’s airway as it copes with more than 500 liters of air that are filtered hourly into the lung. During this time it is thought that more than 25 million particles are processed by this epithelium [34, 35]. Mucociliary activity removing mucus towards the nasopharynx, immunological activities involving a variety of immunocompetent cells and metabolism of endogenous substances are further essential functions of the nasal structures. The nasal cavity connected to other cavities such as the frontal and maxillary sinus and the ear also serves as a resonant body.
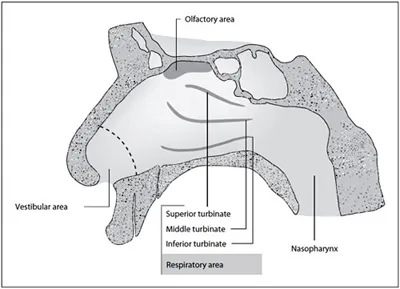
Fig. 1. Sagittal section of the nasal cavity.
There are 3 distinct functional areas (fig. 1) in the nasal cavity, the vestibular, olfactory and respiratory zones. The vestibular area (approx. 0.6 cm2) serves as a first barrier against airborne particles with low vascularization comprised of stratified squamous and keratinized epithelial cells with nasal hairs. The olfactory area (approx. 15 cm2) enables olfactory perception and is highly vascularized. The respiratory area (approx. 130 cm2) serves with its mucus layer produced by highly specialized cells as an efficient air-cleansing system [36]. The surface of this zone is enlarged by the division of the cavity by lateral walls into 3 nasal conchae or turbinates and by the magnification of the mucosa by microvilli and cilia. The magnification in terms of square centimeters is unknown. The zone is highly vascularized. The posterior region of the nasal cavity is the nasopharynx. Its upper part consists of ciliated cells, the lower part contains squamous epithelium. The area is also part of the mucosal immune system.
Due to the rich vascularization, the olfactory and in particular the respiratory zone may serve as an efficient absorption surface for topically applied drugs. The olfactory region with its vicinity to the cerebrospinal fluid and direct nervous interface to the brain has attracted research interest for possible nose-to-brain delivery.
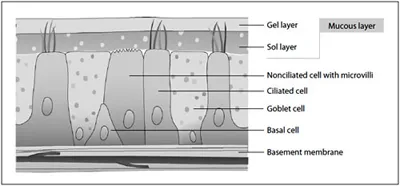
Fig. 2. Cell types of the nasal epithelium with covering mucous layer.
The respiratory epithelium as well as other parts of the nasal cavity and airways are lined by superficial epithelium (fig. 2) consisting primarily of 2 types of cells: mucus-producing goblet cells (20%) and ciliated cells (80%). The various cell types of the epithelium are joined together by tight junctions. Mucus continuously produced by goblet cells traps inhaled particulate and infectious debris while the propulsive force (about 1,000 strokes/min) generated by ciliated cells transports the mucus towards the nasopharynx and the gastrointestinal tract for elimination. This effective cleansing mechanism is called mucociliary clearance (MCC) [37]. The MCC time is approximately 20 min but is subject to great inter-subject variability. The MCC is dependent on the function of the cilia and the characteristics of the covering mucus, which can be influenced by acute or chronic illnesses like common cold or allergic rhinitis. Many substances can influence the MCC of the airways, either by stimulation or inhibition. A stimulatory effect of drugs on the MCC is of clinical importance, because these substances can possibly be used to improve pathological conditions of the MCC. Components (drug, ingredient) of nasally administered formulations with a too pronounced MCC-impairing activity may limit their use.
Nasal Delivery
The intranasal administration represents a viable option for local and systemic delivery of many therapeutic agents. Therapeutic and pharmaceutical considerations direct the development of nasal products [38].
Therapeutic Considerations
Answers to key questions whether the drug is intended for (a) local or systemic delivery or for (b) single or repetitive administration and (c) patient-related issues (e.g. adults, children) define the development strategy for the nasal product. An idea of the clinically effective drug concentration in the target site should exist in order to estimate the feasibility of the nasal application route.
Local Delivery
Prominent examples for locally acting intranasally administered drugs are decongestants for nasal cold symptom relief, antihistamines and corticosteroids for allergic rhinitis. Due to the fact that relatively low doses are effective when administered topically, the intranasal administration of antihistamines and corticosteroids has a weak potential for systemic adverse effects as opposed to systemic therapy. Intranasal administration is therefore a logical delivery choice for the topical (local) treatment of nasal symptoms.
Systemic Delivery
The nasal mucosa provides a practical entrance portal for systemically acting molecules. Intranasal administration offers a rapid onset of therapeutic effects, avoids the first-pass effect or gastrointestinal degradation of drugs, is noninvasive, essentially painless and finally easily administered by patients or by physicians in emergency settings. The intranasal administration provides a true alternative route for systemic drugs presently delivered more conventionally by oral or parenteral routes.
Single versus Repetitive Administration
The disease, the therapeutic goal and the therapeutic agent predefine the dosing regimen. Dosing frequencies of currently marketed intranasally administered products range from weekly dosing to multiple times daily. To avoid multiple parenteral applications, repetitive intranasal administration may be practical for the situation of chronic application with orally insufficient drug bioavailability. The delivery target (local, systemic) as well as the intended dosing schedule govern the development strategy and therefore predefine the drug form (dissolved, ionized etc.), the vehicle form (solid, semisolid, liquid) including the specific ingredients to form the vehicle system (powder, gels, microspheres, solution etc.) and the application device, which determines the drug de...