![]()
Treatment
Bandello F, Zarbin MA, Lattanzio R, Zucchiatti I (eds): Management of Diabetic Retinopathy.
Dev Ophthalmol. Basel, Karger, 2017, vol 60, pp 63-70 (DOI: 10.1159/000460496)
______________________
Intravitreal Ranibizumab in Diabetic Macular Edema: Long-Term Outcomes
Ilaria Zucchiatti · Francesco Bandello
Department of Ophthalmology, Vita-Salute University, Scientific Institute San Raffaele, Milan, Italy
______________________
Abstract
Intravitreal ranibizumab (RBZ) has been shown in multiple randomized clinical trials to be a valuable treatment for diabetic macular edema (DME), promoting a significant improvement in best-corrected visual acuity (BCVA) and in anatomic outcomes. Compared to sham (RISE and RIDE studies), RBZ rapidly and sustainably improved BCVA and decreased macular edema at 2 years, reducing the risk of further vision loss, with low rates of local or systemic side effects. Compared to macular laser photocoagulation (READ-2 study), RBZ provided a greater improvement in BCVA and regression in foveal thickness, but required a higher number of injections compared to patients treated with both RBZ and laser. In RESTORE trial, RBZ alone or combined with macular laser turned out to be superior to laser alone, without significant differences between the 2 RBZ groups. Compared to combined treatment (RBZ or triamcinolone associated with macular laser) or photocoagulation laser alone (Diabetic Retinopathy Clinical Research Network trial), RBZ with prompt or deferred laser was more effective than laser alone at 1-year follow-up. At 3 years, prompt laser was not better than deferring laser for 24 weeks or more. At 5 years, subjects treated with RBZ achieved better long-term visual outcomes than patients managed with triamcinolone or laser followed by very deferred RBZ. In conclusion, randomized clinical trials showed that RBZ was superior to laser in DME treatment, providing excellent long-term visual outcomes. Frequent injections were necessary in most of the patients to properly control DME and maximize the visual benefits.
© 2017 S. Karger AG, Basel
Vascular endothelial growth factor (VEGF) has a crucial role in the development and progression of diabetic macular edema (DME). This homodimeric protein is implicated in vascular permeability and neovascularization, promoting vascular endothelial cell growth. Increased levels of VEGF have been identified in the anterior and vitreous chambers, correlating with DME severity [1, 2]. A number of clinical trials demonstrated that VEGF is an important therapeutic target for DME. Different classes of VEGF inhibitors are currently used, including ranibizumab (Lucentis®), bevacizumab (Avastin®), pegaptanib sodium (Macugen®), and aflibercept (Eylea®).
Ranibizumab (Lucentis®, Genentech, Inc., South San Francisco, CA, USA) is a humanized monoclonal antibody fragment designed to bind all active isoforms of VEGF-A and their biological degradation products [3]. Ranibizumab (RBZ) is currently approved in United States and European Union for the treatment of DME. Several randomized clinical trials have analyzed the effectiveness and safety of intravitreal RBZ alone or adjunctive to laser photocoagulation in DME treatment (Fig. 1, 2).
The READ-1 study published in 2006, the earliest results regarding intravitreal RBZ (0.5 mg) as a monotherapy compared to sham, showed in a few patients a significant reduction in central foveal thickness (CFT) and a meaningful improvement in best-corrected visual acuity (BCVA) [4].
In 2010, the RESOLVE study, a 12-month multicenter clinical trial, compared the effects of intravitreal RBZ (0.3 or 0.5 mg) to sham therapy [5]. Randomized patients underwent 3 monthly injections and were then retreated if needed, on a pro-re-nata (PRN) regimen. Rescue laser was offered according to protocol-defined criteria. After month-1, the subjects could receive a dose-doubling RBZ injection if they met the per-protocol-specified criteria. At 12 months, patients treated with RBZ experienced a significant improvement in BCVA (10.3 letter increase) compared to sham (1.4 letter decrease) and a meaningful reduction in central retinal thickness (CRT). Sixty percent of patients treated with RBZ experienced an increase of 10 or more letters, compared to 18% of placebo patients. In addition, intravitreal RBZ showed a safety profile consistent with previous studies.
In 2012, intravitreal RBZ (0.5 or 0.3 mg) was compared to sham injections in the RISE and RIDE studies [6]. Macular laser was considered as a rescue treatment according to defined criteria. At 24-month follow-up, patients treated with RBZ achieved a rapid and sustained improvement in vision and also a reduction in macular edema compared to sham. In the RIDE study, 33 and 45% of patients treated respectively with low and high RBZ dosing experienced a visual improvement of ≥15 letters compared to 12% of placebo subjects. In the RISE study, the percentage of patients that gained ≥15 letters was 45% in 0.3 mg RBZ group and 39% in 0.5 mg RBZ group versus 18% in the sham cohort. Low rates of local or systemic side effects were noted, consistent with prior RBZ studies. Moreover, patients treated with RBZ underwent fewer macular laser procedures compared to sham.
Recently, an analysis of data of RISE and RIDE studies evaluated the BCVA and retinal thickness outcomes of subjects with DME and delayed anatomic response after 3 RBZ injections over 24 months [7]. Patients included in the study were divided into 3 groups: immediate responders (patients treated with RBZ who showed more than 10% reduction in CFT after 3 injections), delayed responders (patients with a reduction of 10% or less), and sham-treated patients. The study revealed that 9-10% of RBZ-treated eyes were considered delayed responders. At 24 months, delayed responders turned out to have less CFT reduction but a similar number of letters gained from baseline and similar improvement in diabetic retinopathy (DR) severity compared to immediate responders. This analysis demonstrated that a small number of patients might be slow to respond anatomically, but they still could achieve vision improvement and DR improvement comparable to immediate responders with continued RBZ injections over 24 months of follow-up.
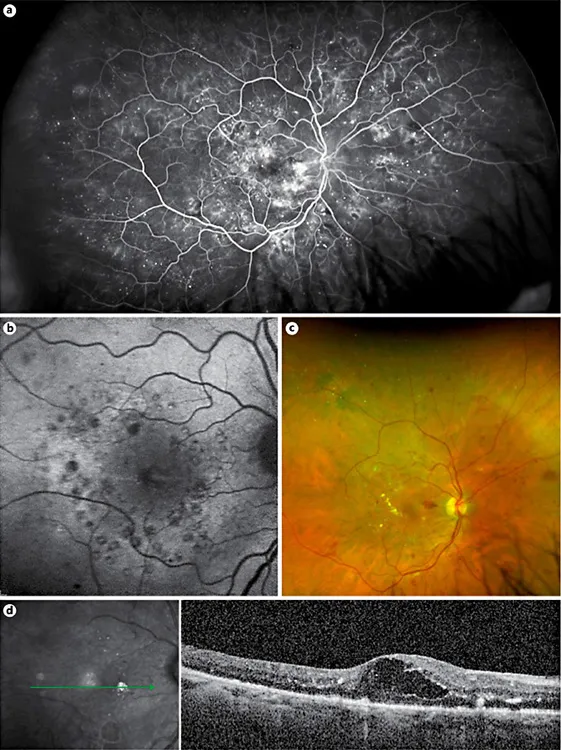
Fig. 1. A 68-year-old man with type-2 diabetes mellitus and poor glycemic control came for follow-up. Macular laser was performed 4 months earlier in the right eye. Nevertheless, the patient still complained of persistent metamorphopsia and low vision in the right eye; the best-corrected visual acuity was 20/63. a Ultra-wide-field fluorescein angiography of right eye showing a breakdown of the blood-retinal barrier, macular laser spots and localized areas of retinal ischemia. Autofluorescence (b) and color image (c) disclosed the presence of laser spots, retinal hemorrhages, hard exudates, and macular edema. d SD-OCT scan revealed macular edema with large intraretinal cysts and hard exudates.
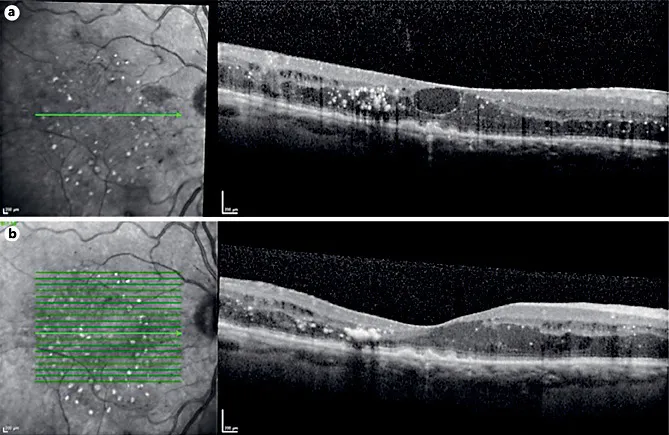
Fig. 2. Follow-up of the same patient of Figure 1 treated with intravitreal ranibizumab (RBZ) injections. a After 3 intravitreal injections of RBZ (loading phase), the best-corrected visual acuity (BCVA) showed a mild improvement to 20/50. SD-OCT examination revealed the persistence of macular edema with a large central intraretinal cyst and hard exudates. b At 9-month follow-up, after a total of 5 intravitreal injections, SD-OCT showed flattening of the retina with persistence of small intraretinal cysts and hard exudates. BCVA was 20/40.
Predictors of the frequency of RBZ injections for DME treatment in the open-label extension were identified [8]. Patients with less advanced disease at baseline received less frequent injections, responding better to RBZ.
The effect of RBZ on hard exudate resolution has been assessed in a recent exploratory analysis of RISE and RIDE studies, with RBZ-treated patients showing a greater reduction of hard exudates compared to sham controls [9]. The reabsorption of hard exudates was slower and more gradual than fluid resolution; nevertheless, the total area of hard exudates did not increase after treatment.
A recent post-hoc analysis of the RIDE and RISE trials evaluated the effects of systemic factors on vision changes from baseline in DME patients treated with RBZ [10]. The study revealed that the BCVA improvement with RBZ was not influenced by systemic factors, including diabetes medication history, serum glucose, glycosylated hemoglobin, blood pressure, body mass index, and renal function. This study suggested that the visual response to RBZ in DME was not influenced by systemic factors related to the management of diabetes.
The READ-2 randomized clinical trial compared RBZ with focal/grid laser photocoagulation or a combination of both in patients with DME [11, 12]. At 6 months (primary end-point), patients treated with intravitreal RBZ injections showed a significantly better outcome in BCVA (+7.24 letters) than those treated with only macular laser photocoagulation (-0.43 letters) [5]. Patients included in the combination group did not achieve a statistically significant improvement in BCVA (+3.80 letters) compared to other groups. After the primary end-point, most subjects in all groups were treated only with RBZ. At 2 years, intravitreal RBZ continued to show positive effects in terms of BCVA gain or CFT reduction [12]. In addition, in the combination group, the amount of residual edema and the frequency of required injection were lower compared to other groups. The mean number of injections was 5.4, 4.4, and 2.9 in RBZ, laser, and combined groups, respectively during the 18 months following the primary end-point. The authors concluded that RBZ alone or with additional laser seemed to be more effective than macular laser alone. Later, the extended study [13] evaluated visual and anatomical outcomes at 3 years, analyzing also the need for prolonged frequent retreatment. Patients treated only with RBZ showed a further improvement in BCVA at month-36, compared to month-24 (10.3 vs. 7.2 letters) and an extra regression in CFT. The RBZ plus laser and the laser alone groups did not achieve a significant variation in terms of BCVA and CFT at year-3 compared to year-2. The mean number of injections was greater in the RBZ group (5.4) compared to laser alone (2.3 injections) and the RBZ + laser ...