![]()
Chapter 1
Surface Analysis Techniques for Dental Materials
Edward Sacher*,‡ and Rodrigo França†,§
*Regroupement Québecois de Matériaux de Pointe,
Département de Génie Physique, École Polytechnique de Montréal,
C.P. 6079, Montréal, Québec H3C 3A7, Canada
†Department of Restorative Dentistry, Dental Biomaterials
Research Laboratory, College of Dentistry, University
of Manitoba, 780 Bannatyne Av, Winnipeg,
Manitoba R3E 0W2, Canada
For many years, dental materials researchers have made significant efforts to understand the chemical and mechanical behaviors of restorative materials, implants, etc., to assure positive interaction with oral tissues. The main focus of these studies has been on bulk properties. However, all reactions between dental materials and the host tissues occur at the interface between their two surfaces. Bulk and surface physicochemical compositions may be quite different. Consequently, meticulous surface characterizations should be carried out on dental materials to better comprehend their behaviors in such situations.
1.Introduction
Information on the chemistry, crystal structure, size, roughness, etc., of a surface is often needed by the dental researcher. There are techniques available today that will provide such information. However, these techniques are often in the domain of chemists and physicists, whose primary interests lie outside the area of dental research; dental researchers, who have little background in the physics, chemistry, or mathematics of such techniques, may be loath to use them for that reason, despite the potential benefits. It is the purpose of this chapter to acquaint them with several of the more common surface characterization techniques, and what they will be able to achieve by using them.
This chapter is designed to inform the dental researcher what information any given technique is capable of offering when carried out by a surface scientist competent in that technique. While it is not necessary for the dental researcher to have expertise in physics, chemistry, and mathematics, they are the major pillars on which biomaterial surface research is constructed, and there is no reason why the dental researcher should not avail himself of such aid.
1.1.Surface and Interfaces
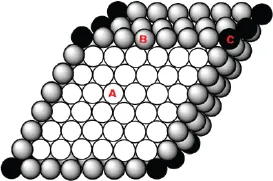
A surface is the boundary layer of a solid or liquid; in equilibrium, when exposed to air or vapor; the surface is generally pictured as having length and width, but no depth. In fact, all real surfaces have some minimal depth. This is because, in the interior, an atom is surrounded uniformly, with the interaction energy of that atom (A in Figure 1) and its neighbors uniformly distributed; however, at the surface (B in Figure 1), the energy distribution is not uniform, due to the lack of structural uniformity (i.e. neighbors lying below, but not above, the surface atoms). This generally results in slight structural changes at the surface. Since structural interactions extend beyond nearest neighbors, the energy distribution at the surface does not change abruptly over one monolayer, but over several, creating a surface with some depth, over which conditions change more gradually.
Figure 1.Cross-section representation of the atomic arrangement of a biomaterial. Bulk atoms (A) are in equilibrium, while surface atoms (B and C) have an additional surface free energy
An interface is a boundary between two condensed phases in equilibrium: solid–solid, solid–liquid, or two immiscible liquids. The importance of the interface is due to the fact that this is where all reactions between the biomaterial and the biological environment occur. In dentistry, phenomena such as osseointegration and dentin adhesion occur, at the molecular level, at the interface. At this level, all materials exposed to air adsorb gases, which form a thin superficial layer. In the same way, dental materials, when immersed in biological fluids (saliva, blood, etc.), are immediately covered by biomolecules, such as water, sugars, and proteins. Such adsorption is driven by the thermodynamic need to reduce the surface free energy.
1.2.Surface Energy
In order to create a surface, energy must be applied to a volume, by breaking bonds and interrupting interactions; that is, the formation of a surface is energetically unfavorable. For liquids, the surface energy density (energy per unit area of surface) is identically equal to the surface tension (force necessary to open a unit length of crack to form the surface). For solids, unless there is some drastic chemical or physical change that occurs on surface development, they are essentially equal.
Thermodynamics informs us that, in three dimensions, physical or chemical reactions will occur spontaneously when the change in Gibbs free energy is negative. In a similar fashion, in two dimensions (i.e. at a surface), reactions will occur spontaneously when the change in Helmholtz free energy, which is directly related to the surface tension, is negative. This is why, for example, iron rusts: the oxidation that occurs leads to a surface product having a lower surface tension than does the unoxidized surface.
1.3.Forces at Interfaces
Interatomic forces within materials, and across their interfaces, can be both polar and non-polar (dispersive). Also, they can be strong or weak. Polar interactions involve permanent dipoles (or multipoles) across an interface, while dispersive interactions involve instantaneous dipoles (i.e. van der Waals interactions). Polar interactions are by far the stronger of the two. Both types of interaction are invariably present, even in highly polar or highly dispersive materials.
In the case of non-crosslinked polymeric filler materials, polar groups are often able to reorient to some extent. For the most used dental adhesive monomers, hydroxyethyl methacrylate (HEMA), this permits the adhesive surface to exhibit a low surface energy to air (polar groups oriented to provide a highly dispersive surface) and a low interfacial energy in water (polar groups oriented to provide a highly polar surface).
1.4.Reactions at Surfaces: Adsorption
Adsorption is the exposure to, and adhesion of, species from the surrounding medium to the surface. In the formation of rust, water vapor and oxygen from the surrounding atmosphere adsorb onto the metal surface within a very short time following exposure, subsequently reacting to form a mixed hydrated oxide/hydroxide which, when enough Fe2O3·H2O is formed, becomes red rust.
Two areas of importance to the dental profession, which bear on adsorption, are adhesion and biocompatibility.1 Common examples of adsorption in dentistry are the wettability and adsorption of saliva on enamel2 or a denture acrylic base3 and the passivation layer formed in titanium implants4 or basic alloys (NiCr and CoCr).
The adsorption of biomolecules and proteins on surfaces has been noted as a major factor for understanding the biocompatibility of biomaterials.5,6 Indeed, what distinguishes a biomaterial from other materials used in engineering is the fact that the body reacts tolerantly to them and negatively to the others.7 All of these biochemical reactions take place at the biomaterial surface, and the a...