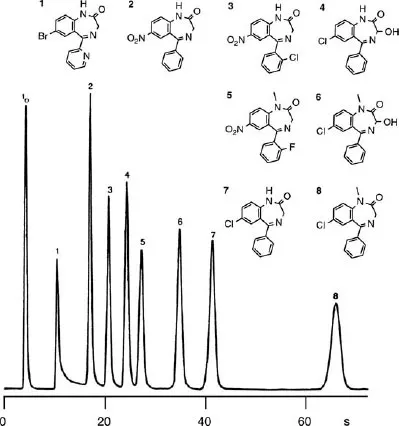
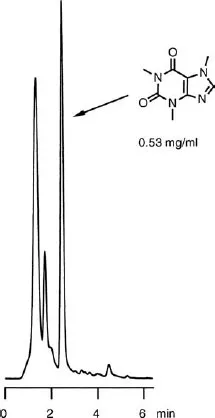
Practical High-Performance Liquid Chromatography
Veronika R. Meyer
- English
- ePUB (disponibile sull'app)
- Disponibile su iOS e Android
Practical High-Performance Liquid Chromatography
Veronika R. Meyer
Informazioni sul libro
Jump into the HPLC adventure!
Three decades on from publication of the 1 st German edition of Veronika Meyer's book on HPLC, this classic text remains one of the few titles available on general HPLC aimed at practitioners.
New sections on the following topics have been included in this fifth edition:
- Comparison of HPLC with capillary electrophoresis
- How to obtain peak capacity
- van Deemter curves and other coherences
- Hydrophilic interaction chromatography
- Method transfer
- Comprehensive two-dimensional HPLC
- Fast separations at 1000 bar
- HPLC with superheated water
In addition, two chapters on the instrument test and troubleshooting in the appendix have been updated and expanded by Bruno E. Lendi, and many details have been improved and numerous references added.
A completely new chapter is presented on quality assurance covering:
- Is it worth the effort?
- Verification with a second method
- Method validation
- Standard operating procedures
- Measurement uncertainty
- Qualifications, instrument test, and system suitability test
- The quest for quality
Reviews of earlier editions
"That this text is written by an expert in both the practice and teaching of HPLC is evident from the first paragraph....not only an enjoyable, fascinating and easy read, but a truly excellent text that has and will serve many teachers, students and practitioners very well." — The Analyst
"…provides essential information on HPLC for LC practitioners in academia, industry, government, and research laboratories…a valuable introduction." - American Journal of Therapeutics
Domande frequenti
Informazioni
1
Introduction
1.1 HPLC: A POWERFUL SEPARATION METHOD
1.2 A FIRST HPLC EXPERIMENT