![]()
1
Basic Principles of Preformulation Studies
1.1 Introduction
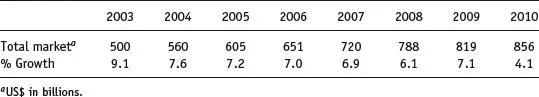
The worldwide market for pharmaceutical sales is large and has grown consistently year-on-year for much of the past decade (Table 1.1). The advent of computer-based drug design programmes, combinatorial chemistry techniques and compound libraries populated with molecules synthesised over many decades of research and development means there is a vast array of compounds with the potential to become drug substances. However, drug substances are not administered to patients as pure compounds; they are formulated into drug products. The selection of a compound, its development into a drug substance and, ultimately, drug product is a hugely time-consuming and expensive process, which is ultimately destined for failure in the majority of cases. As a rough guide, only 1 out of every 5–10 000 promising compounds will be successfully developed into a marketed drug product and the costs involved have been estimated at ca. $1.8 billion (Paul et al., 2010).
Table 1.1 Total market sales in the pharmaceutical sector from 2003 to 2010 (data from IMS Health).
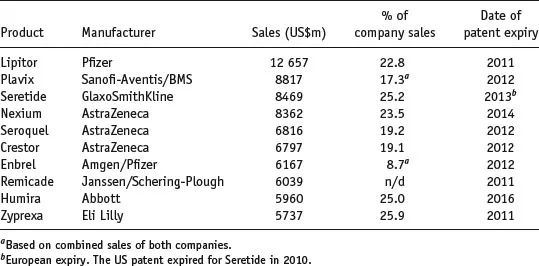
While it is tempting to assume that all drug products are financial blockbusters, approximately 70% never generate sufficient sales to recoup their development costs. Table 1.2 shows the top 20 medicines by sales worldwide (and the percentage of revenue they generate for their respective companies). It is apparent that a significant percentage of income is generated from these blockbuster products, and the financial health and prospects of the originator company are largely dependent upon the extent of patent protection (allowing market exclusivity) and new drug products in the development pipeline.
Table 1.2 Top ten drugs by sales worldwide in 2010 (data from IMS Health).
These numbers imply that development of a drug product in the right therapeutic area can result in significant income, but the costs involved in reaching market are such that only a few potential drug substances can be considered for development. How best to select a compound for development from the myriad of chemical structures that may be available? It is tempting to think that the decision reduces to efficacy against a biological target alone, but in practice physicochemical properties affect how a substance will process, its stability and interaction with excipients, how it will transfer to solution and, ultimately, define its bioavailability. The compound showing greatest efficacy may not ultimately be selected if another compound has a better set of physicochemical properties that make it easier to formulate and/or manufacture. It follows that characterising the physicochemical properties of drug substances early in the development process will provide the fundamental knowledge base upon which candidate selection, and in the limit dosage form design, can be made, reducing development time and cost. This is the concept of preformulation.
1.2 Assay design
In the early stages of preformulation the need rapidly to determine bioavailability, dose and toxicity data predominate and hence the first formulations of a drug substance are usually for intravenous injection. The first task facing any formulator is thus to prepare a suitable formulation for injection – most often this requires only knowledge of solubility and the development of a suitable assay. It is extremely important to note here that no development work can proceed until there is a suitable assay in place for the drug substance. This is because experimentation requires measurement.
1.2.1 Assay development
Assays greatly assist quantitative determination of physicochemical parameters. Since each assay will in general be unique to each drug substance (or, more correctly, analyte) development of assays may be time-consuming in cases where many drug substances are being screened. The first assays developed should ideally require minimum amounts of sample, allow determination of multiple parameters and be applicable to a range of compounds. For instance, a saturated solution prepared to determine aqueous solubility may subsequently be used to determine partition coefficient, by addition of n-octanol.
Note at this stage that determination of approximate values is acceptable in order to make a go/no go decision in respect of a particular candidate and so assays do not need to be as rigorously validated as they do later in formulation development. Table 1.3 lists a range of molecular properties to be measured during preformulation, in chronological order, and the assays that may be used to quantify them. These properties are a function of molecular structure. Once known, further macroscopic (or bulk) properties of the drug candidate can be measured (Table 1.4). These properties result from intermolecular interactions. Note also that determination of chemical structure does not appear, as it is assumed that the chemists preparing the candidate molecules would provide this information. Note also that solubility will be dependent upon physical form (polymorph, pseudopolymorph or amorphous).
Table 1.3 Molecular sample properties and the assays used to determine them.
| Solubilitya | UV | Chromophore |
| pKa | UV or potentiometric titration | Acid or basic group |
| Po, w/log P | UV
TLC
HPLC | Chromophore |
| Hygroscopicity | DVS
TGA | No particular requirement |
Stability - Hydrolysis
- Photolysis
- Oxidation
| HPL... |