Organosilicon Chemistry
Novel Approaches and Reactions
Tamejiro Hiyama, Martin Oestreich, Tamejiro Hiyama, Martin Oestreich
- English
- ePUB (disponibile sull'app)
- Disponibile su iOS e Android
Organosilicon Chemistry
Novel Approaches and Reactions
Tamejiro Hiyama, Martin Oestreich, Tamejiro Hiyama, Martin Oestreich
Informazioni sul libro
Provides a unique summary of important catalytic reactions in the presence of silicon A must-have for all synthetic chemists, this book summarizes all of the important developments in the application of organosilicon compounds in organic synthesis and catalysis. Edited by two world leaders in the field, it describes different approaches and covers a broad range of reactions, e.g. catalytic generation of silicon nucleophiles, Si-H Bond activation, C-H bond silylation, silicon-based cross-coupling reactions, and hydrosilylation in the presence of earth-abundant metals. In addition to the topics covered above, Organosilicon Chemistry: Novel Approaches and Reactions features chapters that look at Lewis base activation of silicon Lewis acids, silylenes as ligands in catalysis, and chiral silicon molecules. -The first book about this topic in decades, covering a broad range of reactions
-Covers new approaches and novel catalyst systems that have been developed in recent years
-Written by well-known, international experts in the areas of organometallic silicon chemistry and organosilicon cross-coupling reactions Organosilicon Chemistry: Novel Approaches and Reactions is an indispensable source of information for synthetic chemists in academia and industry, working in the field of organic synthesis, catalysis, and main-group chemistry.
Domande frequenti
Informazioni
1
Catalytic Generation of Silicon Nucleophiles
1.1 Introduction

1.2 Silicon Nucleophiles with Copper Catalysts
1.2.1 Copper‐Catalyzed Nucleophilic Silylation with Disilanes
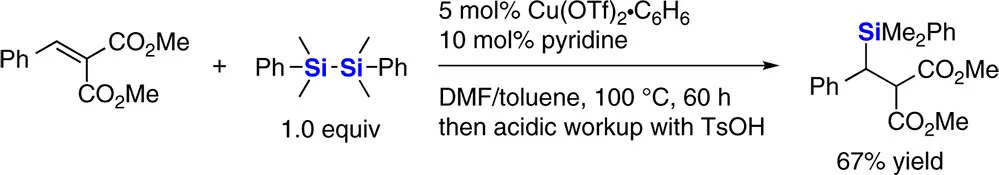
1.2.1.1 Silylation of α,β‐Unsaturated Carbonyl Compounds

1.2.1.2 Silylation of Alkylidene Malonates