In this chapter, apart from brief general remarks, some aspects of enzymes and enzyme inhibitors employed for therapeutic applications will be discussed. The topics are, among others, the following:
• The world market of these drugs
• Regulatory authorities (mainly the US FDA and the EMA)
• Quality standards
• Rare diseases and orphan drugs
• Therapeutic enzymes and enzyme inhibitors
• Cost of drug development and therapies
• Future developments
The term “drug” is used here in connection with recombinant therapeutic proteins (for the world market of therapeutic proteins, see Research and Markets, 2016) as well as chemically synthesized small molecules that have been approved for the treatment and prevention of diseases. Not included are diagnostic imaging agents as, e.g., Netspot (detection of rare neuroendocrine tumors) or Axumin (detection of recurrent prostate cancer) both approved by the FDA in 2016, and other compounds used for diagnostic purposes. The targets of small molecule drugs are proteins to which they bind to exert therapeutic efficacy; enzymes involved in drug metabolism are not defined here as drug target. The WHO Collaborating Centre for Drug Statistics Methodology classifies drugs by The Anatomical Therapeutic Chemical (ATC) Classification System (ATC and ATCvet) with respect to the organ or system, on which they act, their therapeutic, pharmacological, and chemical properties, etc., including, e.g., defined daily drug doses (WHOATC 2018).
Enzyme inhibitor drugs are used to treat chronic diseases and disorders such as cardiovascular diseases (angina and myocardial infarction, heart failure, hypertensive heart and rheumatic heart disease, etc.), gastrointestinal diseases, Diabetes Type 2, pain, fever, inflammation, neurodegenerative diseases such as Parkinson’s and Alzheimer’s, asthma and COPD, psoriasis and psoriatic arthritis, different infectious diseases (trypanosomiasis, leishmaniasis, tuberculosis, malaria, acquired immunodeficiency syndrome, etc.), a variety of different types of cancers (see below), some of the rare lysosomal storage diseases, life style related conditions (erectile dysfunction, benign prostatic hyperplasia, alopecia) and many others.
Pharmaceutical enzymes may be categorized as enzymes in replacement therapy, enzymes in cancer treatment, enzymes for fibrinolysis, enzymes to cure digestive disorders and enzymes that are used topically for various treatments (Yari et al., 2017). Modern DNA technology together with other approaches such as PEGlation or glycoengineering generates enzymes with extended half-life and reduced or no immunogenicity.
The Food and Drug Administration (FDA or USFDA) a federal agency of the United States Department of Health and Human Services is—together with several FDA organizations among them the Center for Drug Evaluation and Research (CDER)—responsible for controlling apart from cosmetics, animal foods and feed, tobacco products, dietary supplements and veterinary products, new drug application (new molecular entities, NMEs) and prescription, over-the-counter pharmaceutical drugs, generic drugs, vaccines, biopharmaceuticals, etc. An NME is a drug with an active moiety that is approved by the FDA as a single ingredient drug or as part of a combination product or marketed in the US for the first time; hence, treatment with an NME is often tantamount to a new therapy for patients.
Its European counterpart is the European Medicines Agency (EMA), which recently moved from London to Amsterdam. The function of the EMA (2017, 2018) is to protect “public and animal health in 28 EU Member States, as well as the countries of the European Economic Area, by ensuring that all medicines available on the EU market are safe, effective and of high quality; the EMA serves a market of over 500 million people living in the EU.” A European medicines regulatory network aims among others on reducing the administrative burden through the centralized authorization procedure, translating into medicines reaching patients faster. The EMA has a number of committees for Medicinal Products for Human Use, Pharmacovigilance Risk Assessment, for Medicinal Products for Veterinary Use, for Orphan Medicinal Products, Herbal Medicinal Products, for Advanced Therapies, and a Paediatric Committee.
According to more recent developments, the FDA offers an Expedited Program for speeding up the availability of drugs that treat serious diseases: Fast track (the aim is to facilitate the development, and expedite the review of drugs to treat serious conditions, filling an unmet medical need), breakthrough therapy (substantial improvement over available therapy), priority review (FDA’s goal is to take action on an application within 6 months), and accelerated approval (allows drugs to be approved based on a surrogate endpoint) are the 4 ways applied singly or in conjunction in which the FDA cooperates with the industry (see Table 1.1) in developing drugs (FDA, 2018; 2018a). This resulted in an increase of the speed at which new drugs are and have been developed and reviewed, and is expressed by the fact that 78% and 87% of drug applications were approved by the FDA in the first review cycle in 2014 and 2015, respectively, compared with just 58% between 2008 and 2012 (Tworkoski, 2016).
Table 1.1 Drugs approved by the FDA in 2016 API | Drug Name | FDA-approved use | Expedited program | Company |
Elbasvir & grazoprevir | Zepatier | Chronic hepatitis C virus | BT | MSD SHARP & DOHME GMBH |
Brivaracetam | Briviact | Partial onset seizures | | UCB (Union Chimique Belge) |
Obiltoxaximab | Anthim | Inhalational anthrax | | Elusys Therapeutics, Inc. |
Ixekizumab | Taltz | Plaque psoriasis | | Eli Lilly & Company |
Reslizumab | Cinqair | Severe asthma | | Teva Pharmaceuticals |
Defibrotide sodium | Defitelio | Hepatic veno-occlusive disease | PR, OD | Jazz Pharmaceuticals |
Venetoclax | Venclexta | Chronic lymphocytic leukemia | AA, BT, PR, OD | AbbVie Inc. |
Pimavanserin | Nuplazid | Hallucinations & delusions | BT, PR | Acadia Pharmaceuticals Inc. |
Atezolizumab | Tecentriq | Urothelial carcinoma | AA, BT, PR | Genentech |
Daclizumab | Zinbryta | Multiple sclerosis | | Biogen, Inc. |
Obeticholic acid | Ocaliva | Chronic liver disease | AA, FT, OD | Intercept Pharmaceuticals, Inc. |
Fluciclovine F 18; imaging agent | Axumin | To detect recur-rent prostate cancer | | Blue Earth Diagnostics, Ltd. |
Ga 68 dotatate; imaging agent | NETSPOT | To detect neuroendocrine tumors | PR, OD | Advanced Accelerator Applications USA, Inc. |
Sofosbuvir & velpatasvir | Epclusa | All six major forms of hepatitis C virus | PR | Gilead Sciences, Inc. |
Lifitegrast ophthalmic solution | Xiidra | Dry eye disease | | Shire US Inc. |
Lixisenatide | Adlyxin | Improve glycemic control | | Sanofi-Aventis U.S. LLC |
Eteplirsen | Exondys 51 | Duchenne muscular dystrophy | AA, FT, PR, OD | Sarepta Therapeutics |
Olaratumab | Lartruvo | Soft tissue sarcoma | AA, BT, FT, PR, OD | Eli Lilly & Company |
Crisaborole | Eucrisa | Atopic dermatitis | | Anacor Pharmaceuticals, Inc. |
Bezlotoxumab | Zinplava | Decrease the risk of Clostridium difficile infection | | Merck Sharp & Dohme Corp. |
Rucaparib | Rubraca | Ovarian cancer | AA, BT, PR, OD | Foundation Medicine, Inc. |
Nusinersen | Spinraza | Spinal muscular atrophy | FT, PR, OD | Ionis Pharmaceuticals |
Note: AA, Accelerated approval; PR, Priority review status, BT, Breakthrough therapy designation; FT, Fast track designation; OD, Orphan drug designation; API, Active Pharmaceutical ingredient. |
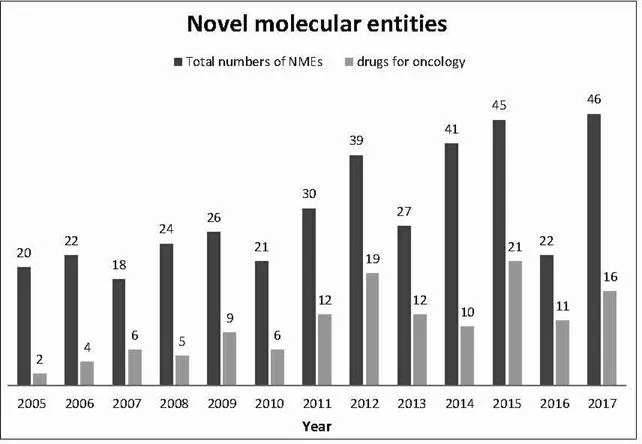
The understanding of cancer pathogenesis has significantly improved in the recent past which has led to new therapeutic concepts such as targeted agents designed to inhibit molecular pathways that are crucial for tumor growth, or immunotherapeutic approaches (Vanneman and Dranoff, 2012; Gotwals et al., 2017; Banchereau and Palucka, 2018) including immune checkpoint inhibitors (Dine et al., 2017). Another recent example is Yescarta, a chimeric antigen receptor (CAR) T cell therapy, approved as the second gene therapy by the FDA for treating diffuse large B-cell lymphoma (DLBCL, the most common type of non-Hodgkin lymphoma; FDA, 2018c); Yescarta has received the orphan drug status (see next section). This has led to an improved survival of patients suffering from certain types of cancer and finds expression, too, in a trend towards an increased share of oncologic drugs as part of the number of drugs approved per year as shown in the following figure (see also Centerwatch, 2018).