
eBook - ePub
Structure
Gengxiang Hu, Xun Cai, Yonghua Rong
This is a test
Condividi libro
- 349 pagine
- English
- ePUB (disponibile sull'app)
- Disponibile su iOS e Android
eBook - ePub
Structure
Gengxiang Hu, Xun Cai, Yonghua Rong
Dettagli del libro
Anteprima del libro
Indice dei contenuti
Citazioni
Domande frequenti
Come faccio ad annullare l'abbonamento?
È semplicissimo: basta accedere alla sezione Account nelle Impostazioni e cliccare su "Annulla abbonamento". Dopo la cancellazione, l'abbonamento rimarrà attivo per il periodo rimanente già pagato. Per maggiori informazioni, clicca qui
È possibile scaricare libri? Se sì, come?
Al momento è possibile scaricare tramite l'app tutti i nostri libri ePub mobile-friendly. Anche la maggior parte dei nostri PDF è scaricabile e stiamo lavorando per rendere disponibile quanto prima il download di tutti gli altri file. Per maggiori informazioni, clicca qui
Che differenza c'è tra i piani?
Entrambi i piani ti danno accesso illimitato alla libreria e a tutte le funzionalità di Perlego. Le uniche differenze sono il prezzo e il periodo di abbonamento: con il piano annuale risparmierai circa il 30% rispetto a 12 rate con quello mensile.
Cos'è Perlego?
Perlego è un servizio di abbonamento a testi accademici, che ti permette di accedere a un'intera libreria online a un prezzo inferiore rispetto a quello che pagheresti per acquistare un singolo libro al mese. Con oltre 1 milione di testi suddivisi in più di 1.000 categorie, troverai sicuramente ciò che fa per te! Per maggiori informazioni, clicca qui.
Perlego supporta la sintesi vocale?
Cerca l'icona Sintesi vocale nel prossimo libro che leggerai per verificare se è possibile riprodurre l'audio. Questo strumento permette di leggere il testo a voce alta, evidenziandolo man mano che la lettura procede. Puoi aumentare o diminuire la velocità della sintesi vocale, oppure sospendere la riproduzione. Per maggiori informazioni, clicca qui.
Structure è disponibile online in formato PDF/ePub?
Sì, puoi accedere a Structure di Gengxiang Hu, Xun Cai, Yonghua Rong in formato PDF e/o ePub, così come ad altri libri molto apprezzati nelle sezioni relative a Technik & Maschinenbau e Werkstoffwissenschaft. Scopri oltre 1 milione di libri disponibili nel nostro catalogo.
Informazioni
Chapter 1 Atomic structure and interatomic bonding
Material is referred to a substance with present or expected future applications for human beings and has been considered as the substantial basis of the national economy. The development of industrial and agricultural production, the science and technology progress, and the improvement of people’s living standards are relying on an extensive sort of materials, such as metals, ceramics, and polymers, having various properties that meet the different requirements. For a long time, people have been studying and investigating the factors that influence the properties of the materials and the ways to improve their performance when using the materials. The researches have shown that the atomic structure of the elements within the material is the fundamental factor that determines the material performance. It includes interactions and combinations among atoms, the spatial distribution and movement of atoms or molecules, and the morphology of atomic clusters and so on. Therefore, the first topic we need to understand is the microscopic structure of the material and its internal construction and microstructure state, so that we can find out ways to improve and develop new materials from its internal contradictions.
A substance is made up of atoms, and an atoms consist of a positively charged nucleus is located in the center of the atom and negative electrons outside the nucleus.
The electronic structure of atoms determines the interatomic bonding of the atoms with each other. Therefore, the understanding of the electronic structure of atoms is not only helpful to classify the materials, but also useful to find out the fundamental principles of physical, chemical, and mechanical properties of materials.
1.1 Atomic structure
1.1.1 Substance construction
As is known to all material is composed of numerous fine particles gathered together in some certain way. These particles may be considered as molecules, atoms, or ions. The molecule is a kind of particle that can exist alone and maintain its chemical properties. The volume of a molecule is small, for example, the diameter of a water molecule is about 0.2 nm. However, the mass of different molecules is different. For instance, H2 is the smallest molecule in the world and its relative molecular mass is only 2; however, a natural polymer compound, such as proteins, has an average relative molecular weight of up to a few millions.
Further analysis showed that the molecule is composed of a number of smaller particles, i.e., atoms. In a chemical reaction, the molecules can be further broken down into the atoms; whereas, the atoms are indivisible. Therefore, an atom is the smallest particle in a chemical reaction. However, the atoms are not the basic particles of the material in quantum mechanics. They have complex structures. The atomic structure directly affects the interatomic bonding.
1.1.2 Structures of atoms
The recent scientific experiments show that the atom is composed of the protons and neutrons, as well as the electrons. The neutron in the nucleus is electrically neutral and the proton carries a positive charge, which possesses exactly the same charge as that of an electron, whose charge is equal to –e (e = 1.6022 × 10−19 C). The electrostatic attraction shows that the negatively charged electrons are tightly bound around the positively charged nucleus. As a whole, an atom is electrically neutral because in an atom the number of protons and electrons is the same.
An atom is volumetrically small. The atomic diameter is about the order of 10−10 m, while the diameter of nucleus is only about the order of 10−14 m. Besides, the atom mass is mainly undertaken by the nucleus, while the surrounded electrons are located within a relatively enormously large space in an atom. The mass of an neutron or an proton is approximately 1.67 × 10−24 g, while the mass of electron is about 9.11 × 10−28 g; thus a proton is almost 1,833 times an electron.
1.1.3 Electronic Structures of atoms
The electrons revolve around the nucleus of an atom, which is just like a “cloud” with a negative charge around the nucleus, so it is called the electronic cloud. The electrons have the duality of the wave and particle. The electronic motion doesn’t have fixed orbits but the position probability of its motion outside the nucleus can be determined through the statistical methods according to the energy levels of electrons. The electrons with a lowest energy usually appear near the nucleus, while the electrons with the highest energy move far away from the nucleus. In quantum mechanics, the basic equation reflecting the motion of the microscopic particles is the Schrodinger’s equation. The wave function obtained by solving the equation describes the state of the electronic movement and the occurrence probability in somewhere outside the nucleus. Videlicet, the spatial position and electron energy in an atom can be determined by the four quantum numbers shown as follows:
(1) Principal quantum number n
It determines the electron energy and its average distance with the nucleus of an atom, i.e., the quantum shell of the electron, as shown in Fig. 1.1. n can only be a positive integer, such as 1, 2, 3, 4,…, and it explicitly represents the quantization of the electron energy in an atom, thus being called the principal quantum number. The principal quantum number (or quantum shell) is represented by a capital letter. For example, n=1 means that the quantum shell has the lowest energy, and it is the closest orbit to the nucleus, named as K-shell, according to the old quantum theory. The numbers 2, 3, 4, … indicate higher energy levels named L-, M-, N-, …, respectively.
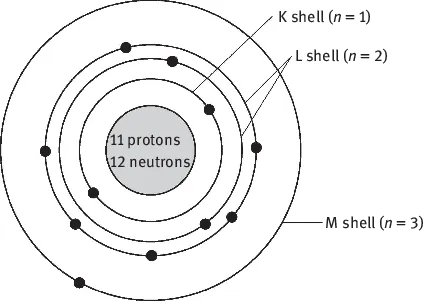
Fig. 1.1: The electron distribution of K-, L- and M-shells in the Na atomic structure.
(2) Azimuthal quantum number li
It indicates the energy level of an electron in the same quantum shell, i.e., the electron subshell. The azimuthal quantum number (also referred to as the secondary quantum number) l can be 0, 1, 2, 3, …. For example, when n equals 2, it means that two orbital azimuthal quantum numbers exist, l2 = 0 and l2 = 1, that is, L-shell consists of two electronic subshells according to the electronic energy difference. For convenience, the electron energycorresponding to the orbit with an azimuthal quantum number of li is commonly labeled as lowercase letters as follows:
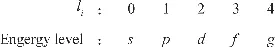
In the same quantum shell, the electron subshell energy is increased by the order of s, p, d, f, and g. The electronic cloud pattern varies in different electronic subshells. For instance, the electronic cloud of s subshell is spherical with the atomic nucleus sitting at the center, and the electronic cloud of p subshell is spindle.
(3) Magnetic quantum number mi
It gives the energy series or orbital number of each azimuthal quantum number. In each electron subshell, the total number of a magnetic quantum number is 2li + 1. If li equals 2, the magnetic quantum number is 2 × 2 + 1 = 5, with the values of −2, −1, 0, + 1, + 2.
Magnetic quantum number determines the spatial orientation of the electron cloud. If the space occupied by the electron cloud has a certain pattern and extension direction in a certain quantum shell, we name it as an orbital, then the four subshells, s, p, d, and f, will have 1, 3, 5, and 7 tracks, respectively.
(4) Spin quantum number si
It reflects different electron spin directions Si is specified as +1/2 and −1/2, which illustrate the spinning clockwise and counterclockwise and are commonly represented by “↑” and “↓”, respectively.
In the nucleus with no less than one electron, the arrangement of electrons outside the nucleus follows the three principles, i.e., the minimum energy principle, the Pauli exclusion principle, and the Hund’s rule.
(a) Minimum energy principle
The elect...