
eBook - ePub
Reactions and Syntheses
In the Organic Chemistry Laboratory
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Reactions and Syntheses
In the Organic Chemistry Laboratory
About this book
The second edition of this classic text book has been completely revised, updated, and extended to include chapters on biomimetic amination reactions, Wacker oxidation, and useful domino reactions.
The first-class author team with long-standing experience in practical courses on organic chemistry covers a multitude of preparative procedures of reaction types and compound classes indispensable in modern organic synthesis. Throughout, the experiments are accompanied by the theoretical and mechanistic fundamentals, while the clearly structured sub-chapters provide concise background information, retrosynthetic analysis, information on isolation and purification, analytical data as well as current literature citations. Finally, in each case the synthesis is labeled with one of three levels of difficulty.
An indispensable manual for students and lecturers in chemistry, organic chemists, as well as lab technicians and chemists in the pharmaceutical and agrochemical industries.
The first-class author team with long-standing experience in practical courses on organic chemistry covers a multitude of preparative procedures of reaction types and compound classes indispensable in modern organic synthesis. Throughout, the experiments are accompanied by the theoretical and mechanistic fundamentals, while the clearly structured sub-chapters provide concise background information, retrosynthetic analysis, information on isolation and purification, analytical data as well as current literature citations. Finally, in each case the synthesis is labeled with one of three levels of difficulty.
An indispensable manual for students and lecturers in chemistry, organic chemists, as well as lab technicians and chemists in the pharmaceutical and agrochemical industries.
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Reactions and Syntheses by Lutz F. Tietze,Theophil Eicher,Ulf Diederichsen,Andreas Speicher,Nina Schützenmeister in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Physical & Theoretical Chemistry. We have over one million books available in our catalogue for you to explore.
Information
Chapter 1
C–C Bond Formation
C–C bond formations are essential for the construction of the backbone of any organic compound, and their mechanistic description can be used as a general tool for their classification. Thus, in Sections 1.1–1.8, the focus is on transformations in which nucleophilic, electrophilic, radical, and pericyclic reactions as well as reactions mediated by organometallics and transition-metal compounds play the decisive role.
In Section 1.1, examples are given of nucleophilic additions to the carbonyl group of aldehydes, ketones, and derivatives of carboxylic acids (esters, anhydrides, etc.) as well as addition to acceptor-substituted olefins (Michael addition) and carbonyl olefination. In Section 1.2, alkylation reactions of aldehydes, ketones, carboxylic acids, and β-dicarbonyl compounds at their α- and γ-positions are described. In Section 1.3, reactions of the aldol and Mannich type and in Section 1.4, electrophilic and nucleophilic acylation reactions are depicted. Section 1.5 deals with reactions of alkenes proceeding via carbenium ions and Section 1.6 with transition-metal-catalyzed reactions such as the Heck reaction and Suzuki–Miyaura, Sonogashira, and metathesis reactions. In Section 1.7, pericyclic reactions such as cycloadditions, electrocyclic transformations, and sigmatropic reactions, and, finally, in Section 1.8 some basic radical reactions are described. Further transition-metal-catalyzed transformations such as the Wacker oxidation are described in Chapters 2 and 5.
1.1 Nucleophilic Addition to Aldehydes, Ketones, Carboxylic Acid Derivatives (Esters, Anhydrides), and α,β-Unsaturated Carbonyl Compounds; Carbonyl Olefination
1.1.1 (E)-4-Acetoxy-2-methyl-2-butenal
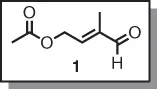 | Topics: |
|
(a) General
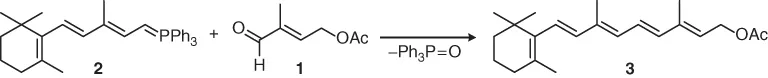
(E)-2-Methyl-2-butenal bearing an acetoxy group at the 4-position can be regarded as a functional isoprene unit and is used as a C5-building block for the synthesis of terpenes by carbonyl olefination [1]. Thus, in the classical industrial vitamin A synthesis of BASF (cf. Section 4.1.5), (E)-4-acetoxy-2-methyl-2-butenal (1) is combined with the C15-ylide 2 in a Wittig reaction to give vitamin A acetate 3:
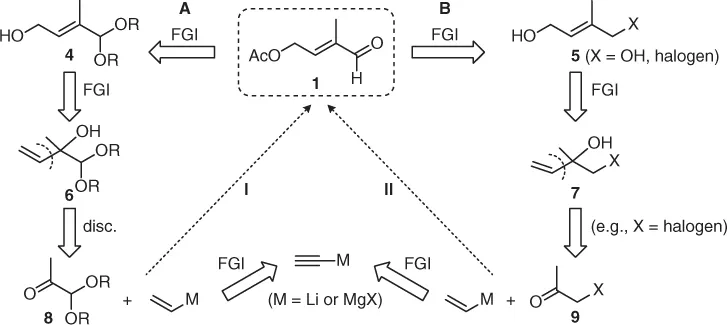
Retrosynthesis of the target molecule 1 can be conducted in two directions (A/B) via the intermediates 4/5 and further by allylic inversions to allyl alcohols 6/7. These should result from the acetone derivatives 8/9 either by addition of allyl metals or by ethynylation followed by partial hydrogenation of the primarily formed acetylenic alcohols (approaches I/II). Both approaches I and II have been described in Refs [2, 3].
App...
Table of contents
- Cover
- Related Titles
- Title Page
- Copyright
- Table of Contents
- Preface to the First Edition
- Preface to the Second Edition
- Acknowledgments
- About the Authors
- Abbreviations and Symbols
- Chapter 1: C–C Bond Formation
- Chapter 2: Oxidation and Reduction
- Chapter 3: Heterocyclic Compounds
- Chapter 4: Selected Natural Products
- Chapter 5: Domino Reactions
- Index of Reactions
- Index of Products
- Subject Index
- End User License Agreement