
eBook - ePub
Focal Therapy in Prostate Cancer
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Focal Therapy in Prostate Cancer
About this book
This book comprehensively reviews the potential of focal therapy and discusses why the changing face of prostate cancer warrants a change in the way we treat men with the disease. It deals with the mechanisms by which disease can be localized within the gland and then the different technologies used for focal ablation. Bringing together eminent contributors in one accessible reference, this book introduces focal therapy to all urologists, oncologists, and radiologists who are involved in the treatment of men with prostate cancer.
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
At the moment all of our mobile-responsive ePub books are available to download via the app. Most of our PDFs are also available to download and we're working on making the final remaining ones downloadable now. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Focal Therapy in Prostate Cancer by Hashim Uddin Ahmed, Manit Arya, Peter R. Carroll, Mark Emberton, Manit Arya,Mark Emberton,Peter R. Carroll,Hashim Uddin Ahmed, Manit Arya, Mark Emberton, Peter R. Carroll, Hashim Uddin Ahmed in PDF and/or ePUB format, as well as other popular books in Medicine & Urology. We have over one million books available in our catalogue for you to explore.
Information
SECTION III
How can we create discrete tissue necrosis?
Chapter 10
Energies for Focal Ablation: Cyroablation
Introduction
Early cryoablative techniques employed a brine (water + salt) cryogen to treat numerous conditions, including headaches and neuralgia. At a concentration of 23.3%, the freezing point of sodium chloride brine is −21°C and that of calcium chloride brine is −40°C. While these temperatures are capable of causing cell injury and death, rapid warming of the brine solution and difficulty in applying these solutions to internally located structures confounded reliable and repeatable tissue targeting and destruction. Between 1845 and 1851, Dr. James Arnott of Brighton, England, used brine solutions to treat cervical and breast tumors reporting both tumor shrinkage and a significant decrease in pain [1]. He later designed an apparatus for the application of cold, which was shown at the Great Exhibition in London in 1851. However, the device was cumbersome to use, had little freezing capability, and found little clinical applicability.
Following World War II, Irving Cooper (a physician) and Arnold Lee (an engineer) collaborated to build a cryosurgical probe that would eventually be the prototype for all subsequent cryosurgical probes. Their design consisted of three long concentric tubes that allowed for closed-loop circulation of a liquid cryogen [2]. The liquefied cryogen was forced at pressure through the inner tube to the tip of the probe, while the space between the inner tube and the middle tube provided a path for the return of the expanded, warmed gaseous form of the cryogen. The third space, between the outer tube and the middle tube, was vacuum insulated with a radiative shield to prevent countercurrent heat exchange. While many cryogens were examined (oxygen, nitrous oxide, carbon dioxide, argon, ethyl chloride, fluorinated hydrocarbons), liquid nitrogen became the cryogen of choice because it is nonflammable, nonexplosive, nontoxic, readily abundant, and inexpensive.
Dr. Cooper introduced these probes for cryogenic surgery of parenchymal lesions through a paper in the New England Journal of Medicine in 1966 [3]. Urologists quickly saw the potential for targeting the prostate with this new instrument and were at the forefront of this renewed interest in cryoablative techniques, with one of the first applications being to treat benign prostatic hyperplasia and subsequently to treat prostate cancer via an open-perineal approach. A closed, transperineal approach with a single cryoprobe that was digitally guided and repositioned as necessary during the procedure to freeze the palpable prostate was introduced in 1974 [4]. At that time, the complications of the closed, transperineal approach were felt to be less debilitating than those associated with a radical prostatectomy, but the approach was poorly accepted because of the difficulty in monitoring cryoprobe placement and ice-ball formation.
The “second generation” of prostate cryoablation began in 1988 when Dr. Onik and colleagues introduced transrectal ultrasound as a real-time guide for monitoring cryotherapy [5]. Again, urologists quickly adopted the procedure for transrectal monitoring of prostate ice formation, and ultrasound gained widespread use through the 1990s when a multiple cryoprobe system was introduced and then a urethral warmer device, which consisted of a closed double-lumen catheter through which heated saline (38–42°C) was continuously circulated with a water pump. Unfortunately, just as the number of patients being treated with prostate cryoablation began to increase, the urethral warmer device produced by Cryomedical Sciences (Kennesaw, GA) was taken off the market for a brief time by the Food and Drug Administration (FDA) to complete a safety and efficacy evaluation. During this time, many serious complications occurred and acceptance of cryotherapy for the treatment of localized prostate cancer met resistance.
The “third generation” of cryoablation originated in 2001 with the introduction of a cryotherapy system based on argon gas cryogen rather than liquid nitrogen. The rapid expansion of argon gas through a small opening within the tip of the cryoprobe cools the tip to −150°C and can be quickly exchanged with helium to induce an active thawing phase (Figure 10.1). This is due to the Joule–Thomson effect in which a temperature change occurs when a gas is forced through a valve and allowed to undergo free expansion in a vacuum.
Figure 10.1 Engineering drawing of gas-driven cryoprobe. Use of gas rather than liquid allowed for smaller needle diameter and subsequently direct prostate access. The Joule–Thomson effect allows the single cryoprobe to both cool and warm the tissue by using Argon and Helium gasses, respectively. (See Plate 10.1.)

At room temperature, all gases except hydrogen, helium, and neon cool upon expansion. Modern cryosystems can use the same Joule–Thomson effect to achieve both cooling with argon gas and tissue warming with helium gas. Since gas can be more easily circulated through a small tube, the cryoprobes (still using the same Cooper design) were reduced in diameter from 3 mm (which required a tissue dilator to place the blunt tipped probe) to the current 1.5-mm (17-gauge) cryoprobes; these cryoprobes have a sharp, pointed design and can be directly inserted into the prostate without tissue dilation using the transperineal approach.
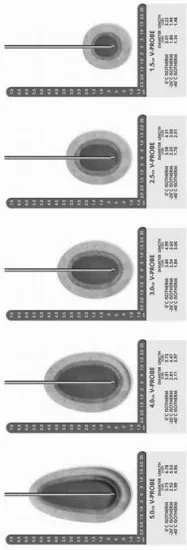
The development of the third generation of cryoablation technology has allowed for the creation of smaller ice zones thereby conforming the ice ball to the individual's unique prostate configuration through pretreatment planning and the placement of multiple cryoprobes. Recently, this concept has been further refined with the introduction of variable ice probes, which allow the user to not only configure the diameter of the ice formation but also its length (Figure 10.2). This evolution of technology has only now allowed us to consider performing organ-preserving prostate cryoablation. The confluence of imaging through transrectal ultrasound, a safe and efficacious urethral warmer, and the reduced needle size all allow variable ice formation that is accurately applied, predictable, and monitored to make organ-preserving therapy possible.
Figure 10.2 Isotherms and ice ball dimensions for cryoprobe. Current technology allows cryoprobes to be individually adjusted to alter the ice ball length and shape. Through careful placement of cryoprobes and adjustment of the ice ball formations, the targeted area for destruction can be selectively treated using these instruments.
Cryobiology
Study of the mechanism of tissue injury (cryobiology) parallels the ebb and flow of clinical practice in cryosurgery, although a much larger body of work exists for cells frozen in the presence of cryoprotective agents for cryopreservation purposes. Understanding the multiplicity of potential mechanisms of injury and death is important to achieve the desired targeted effect with subtotal prostate cryoablation.
Direct cellular injury is the best known and most described mechanism of cellular injury in cryobiology. Direct cellular injury depends on thermal history but occurs through two physical changes: dehydration and ice crystal formation. At low cooling rates, osmotic dehydration occurs as the solute concentration outside the cell increases. This dehydration in turn draws water from the cell, resulting in damage to the enzymatic machinery and a destabilized cell membrane. Alternatively, at rapid cooling rates, the water is trapped inside the cell and results in a super cooling of the cytoplasm and ultimately ice crystal formation that damages both organelles and membranes. These two different mechanisms of direct cell injury result in an inverse curve of cell viability with low viability at extremely rapid and extremely slow cooling rates but high viability at cooling rates between these extremes.
In patients, the cooling and thawing rates are often highly nonlinear and vary throughout the prostate because of tissue heterogeneity and nonuniform heat sinks provided by both vascular structures (dorsal vein complex, neurovascular bundles, prostatic arterioles) and the urethral warmer. However, in general, a lower temperature increases the probability of cell death. Using various prostate cancer cell lines, it has been shown that a decrease in cell viability occurs from about 35% at −20°C to 5% at −40°C. When these lethal end temperatures are achieved, the cooling rate appears to have much less impact on cell viability.
For organ-preserving prostate cryotherapy, monitoring the temperature at the targeted site of destruction is therefore at least as important as monitoring temperatures at vital structures intended for preservation to assure a “lethal” end temperature is achieved. This will theoretically allow slower rates of freezing if necessary to provide the structural preservation desired without compromising oncologic outcome.
Vascular injury is a second but less predictable mechanism by which tissue is both destroyed and preserved with prostate cryoablation. The fact that freezing preferentially destroys the microvasculature over larger blood vessels suggests that cryoinjury affects nutrient and oxygen delivery and may induce necrosis beyond the region of lethal ice formation. This vascular injury has the potential to expand the area of destroyed tissue beyond the target zone. As this effect is less predictable, vascular injury is not used in planning the treatment zone with subtotal prostate cryoablation but may be the source of morbidity related to tissue injury outside the zone of lethal ice.
Direct cellular injury occ...
Table of contents
- Cover
- Title Page
- Copyright
- Contributor List
- Preface to the first edition
- Section I: Is there a role for Focal Therapy in Localised Prostate Cancer?
- Section II: How can we accurately locate cancer within the gland?
- Section III: How can we create discrete tissue necrosis?
- Section IV: How can we determine the success of Focal Therapy?
- Colour plate
- Index