![]()
1
Antioxidants and Radical Scavengers
Abstract:Food antioxidants play an important role in the food industry due to their ability to neutralise free radicals that might be generated in the body. They do that by donating their own electrons to free radicals without becoming free radicals in the process themselves, hence terminating the radical chain reaction. The converted free radical products will then be eliminated from the body before causing any harm; in this regard, antioxidants play the role of scavengers protecting body cells and tissues. In this chapter, the processes which lead to the formation of these reactive species (free radicals) and the different additives used as antioxidants or radical scavengers to counter the effects of free radicals will be discussed. Sources of different types of antioxidants, the various mechanisms by which they work and analytical methods for determination and quality control are also examined.
Keywords: antioxidants free radical species; ORAC assay; HORAC assay; DPPH assay; FRAP assay; Trolox; TEAC assay; ABTS assay; PCL assay; DMPD assay; DL assay; TBARS assay; Brigg-Rauscher assay
1.1 CHEMISTRY OF FREE RADICALS AND ANTIOXIDANTS
1.1.1 Introduction
From the viewpoint of chemistry, free radicals refer to any molecule with an odd unpaired electron in its outer electronic shell, a configuration responsible for the highly reactive nature of such species. The presence of such highly reactive free radicals in biological systems is directly linked to the oxidative damage that results in severe physiological problems. The free radical species that are of concern in living systems include the reactive oxygen species (ROS), superoxide radicals (SOR), hydroxyl radicals and the reactive nitrogen species (RNS). The oxygen-containing reactive species are the most commonly occurring free radicals in living medium and are therefore of greatest concern. The oxidative damage caused by these free radicals can be prevented by using antioxidants which include enzymatic antioxidant systems such as catalase, glutathione peroxidase and superoxide dismutase (SOD) as well as non-enzymatic antioxidants (Figure 1.1). It should be noted that, in nature, the generation of free radicals which cause oxidative stress and that of antioxidants or radical scavengers is carefully controlled such that there is always a balance between the two (Vouldoukis et al. 2004). Examples of non-enzymatic antioxidants include vitamin C (ascorbic acid) which is a sugar acid, vitamin E (α-tocopherol) and β-carotene, bilirubin, propyl gallate (PG, a condensation ester product of gallic acid and propanol), uric acid, tertiary butylhydroquinone (t-BHQ), butylated hydroxyanisole (BHA), ubiquinone and macromolecules which include ceruloplasmin, albumin and ferritin. Generally, mixtures of different antioxidants provide better protection against attack by free radicals rather than individual antioxidants.
Due to the importance of antioxidant systems, there are a number of quality assessment criteria for the antioxidant performance of these systems. Various assays have been developed to assess the antioxidant capacities, including the oxygen radical absorbance capacity (ORAC) assay, ferric reducing ability of plasma (FRAP), Trolox equivalent antioxidant capacity (TEAC) assay, etc. Antioxidant foods which are dietary nutrients containing antioxidant compounds and non-nutrient antioxidants which are normally added to foods to play the role of antioxidants will be discussed simultaneously in this chapter, unless indicated otherwise.
Further Thinking
Free radicals are undesirable due to their instability caused by the electron deficiencies in their structures. They have a high electronic affinity which makes them attack any molecule in their vicinity, generating a chain of reactions which are detrimental to the body and which instigate disorders, diseases, aging and even death.
1.1.2 The formation of ROS in living systems
Under normal conditions, oxygen is vital in metabolic reactions which are necessary for life. Due to its high reactive nature however, oxygen also causes severe damage to living systems due to the generation of reactive oxygen species (ROS; Davies 1995).
The reactive free radicals are generated as part of the energy generation metabolic processes (Raha and Robinson 2000), and are released as a result of a number of reaction procedures in the electron transport chain as well as in the form of intermediate reduction products (Lenaz 2001). Due to the highly reactive nature of free radicals that are formed as intermediates, they prompt electrons to proceed in a concerted fashion to molecular oxygen and thus generate superoxide anion (Finkel and Holbrook 2000). A similar scenario occurs in plants for example, whereby reactive oxygen species are produced during the process of photosynthesis (Krieger-Liszkay 2005).
Examples of reactive species produced as a result of these metabolic reactions include: superoxide anion (O2−), hydrogen peroxide (H2O2), hypochlorous acid and hydroxyl radical (·OH) (Valko et al. 2007). The hydroxyl radicals are known to be unstable; they react spontaneously with other biological molecules in a living medium, causing destructive reactions in foodstuffs and serious physiological damage to consumers (Stohs and Bagchi 1995).
1.1.3 Negative effects of oxidants in food processes and to food consumers
The oxidation process brings about destructive reactions in food items that lead to off-flavour and loss of colour and texture due to the degradation of carbohydrate, protein, vitamins, sterols and lipid peroxidation (Hwang 1991; Pinho et al. 2000; Kranl 2004). The consequences to consumers include damage to nucleic acids, cellular membrane lipids and other cellular organelles, carcinogenesis, mental illnesses and disorders, lung diseases, diabetes, atherosclerosis, autoimmune diseases, aging and heart diseases (Finkel and Holbrook 2000; Lachance et al. 2001; Ou et al. 2002; Yu et al. 2005; Nakabeppu et al. 2006).
1.1.4 Reactive oxygen/nitrogen species and aging
There is strong scientific evidence which relates the reactive oxygen/nitrogen species (ROS/RNS) to aging and pathogenesis (Lachance et al. 2001; Yu et al. 2005; Nakabeppu et al. 2006). In addition, facts have also been presented in many scientific reports that ROS such as peroxyl radicals (ROO·), superoxide ion (O2·+), hydroxyl radicals (HO), etc. play an active role in promoting or inducing numerous diseases such as different types of cancers (Finkel and Holbrook 2000; Ou et al. 2002). Unless these adverse reactions are retarded or prohibited, they will result in food deterioration and health problems to consumers. To counter such harmful effects, antioxidants have been incorporated in many foodstuffs to minimise or solve the problem altogether.
Further Thinking
The incorporation of antioxidants in foodstuffs serves a number of purposes, including the prevention of rancidity phenomena as a result of oxidation (which results in bad odour and off-flavour) of food items containing fats and oils. Antioxidants are also essential in the retention of the integrity of food items (mainly fruits, fruit juices and vegetables) because of their particular properties in preventing browning reactions, extending the shelf life of these food items.
1.2 TYPES OF ANTIOXIDANTS
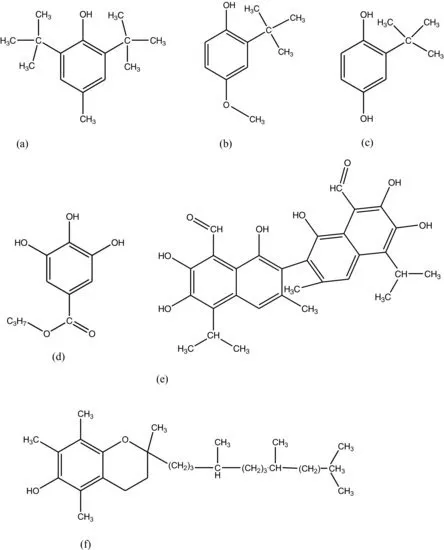
Antioxidants as food additives are used to delay the onset of or slow the pace at which lipid oxidation reactions in food processing proceed. Most of the synthetic antioxidants contain a phenolic functionality with various ring substitutions (monohydroxy or polyhydroxy phenolic compounds) such as butylated hydroxytoluene (BHT), BHA, t-BHQ, PG, gossypol and tocopherol (Figure 1.1). These compounds make powerful antioxidants to protect foodstuffs against oxidative deterioration of the food ingredients. The main chemical attribute that makes them suitable as antioxidants is their low activation energy property, which enables them to donate hydrogen easily and thus put on hold or lower the kinetics of lipid oxidation mechanisms in food systems. The delay to the onset or slowing of the kinetics of lipid oxidation is possible due to the ability of these compounds to either block the generation of free alkyl radicals in the initiation step or temper the propagation of the free radical chain. Due to their positive effects in food processes antioxidants are also known as potential therapeutic agents, thus playing a medicinal role as well. For safety purposes and adherence to quality control standards, the use of any synthetic antioxidant preparation in food processes is expected to meet the following criteria: effective at low concentrations; without any unpleasant odour, flavour or colour; heat stable; non-volatile; and must have excellent carry-through characteristics (Shahidi and Ho 2007).
1.2.1 Natural antioxidants of plant origin
In addition to chemical or synthetic antioxidants, there are also a number of antioxidants that exist naturally in plants and many other herbal materials (Shahidi and Naczk 1995).
Plants that contain natural antioxidants include: carrots, which contain β-carotene and xanthophyll (Chu et al. 2002); ginger roots (Halvorsen et al. 2002); and citrus fruits with their abundance...