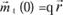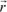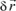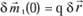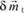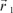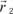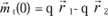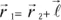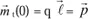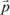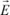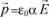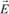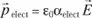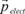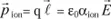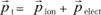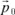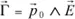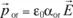![]() PART 1
PART 1
General Physics Phenomena![]()
Chapter 1
Physics of Dielectrics 1
1.1. Definitions
A dielectric material is a more or less insulating material (with high resistivity and with a band gap of a few eV), that is polarizable, i.e. in which electrostatic dipoles exist or form under the influence of an electric field.
Like any material, it is an assembly of ions with positive and negative charges which balance, for a supposedly perfect solid, so as to ensure electrical neutrality. This neutrality is observed at the scale of the elementary structural motifs which constitute solids with ionocovalent bonding (ceramics, for example) and on the molecular scale in molecular solids (polymers and organic solids).
The action of an electric field at the level of these element constituent of solids manifests itself by dielectric polarization effects. Let us remember that the dipole moment of a charge q with respect to a fixed system of reference centered in O is:
where
is the vector which connects the point O to the charge’s position.
If due to a force (caused, for example, by a magnetic field), the charge moves
, then the variation of the moment will be:
represents
the polarization effect of the field on the charge. The generalization of expressions [
1.1] and [
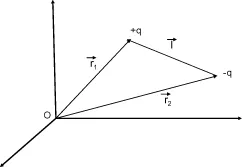
1.2] to a collection of charges occurs by vectorial summation of the moments of each charge. An important case is that of a set of two charges ±q, whose positions are defined by
and
(see
Figure 1.1). The application of [
1.1] to the two charges gives:
is called the dipole moment formed by the two charges, oriented from the negative charge to the positive charge (see
Figure1.1).
The dipole moment appearing in a solid, during the application of a field
, is (to a first approximation) proportional to it. We can then write:
In this equation, α characterizes the polarisability of the species which gave the dipole and e0 the vacuum permittivity.
1.2. Different types of polarization
To study dielectrics, it is necessary to first of all describe the different types of polarization. In order to do so, we must distinguish two types of solids: polar solids and non-polar solids.
1.2.1. Non-polar solids
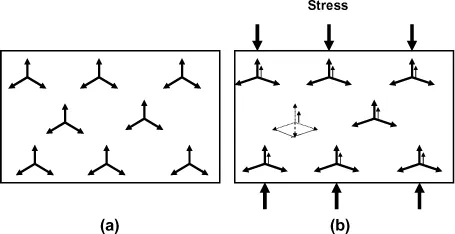
In the case of non-polar solids, the centers of gravity of positive and negative charges coincide, and the dipole moment is therefore null (in the absence of a field). This is the case for solids with metallic bonding, or of numerous ionocovalent solids (ceramic Al2O3, ZrO2, ZnO, SiO2, etc.). Thus, the tetrahedron SiO4 which constitutes the motif of quartz has a null dipole moment. It is the distortion of this tetrahedron, under the effect of a mechanical stress, which will make a polarization and the piezoelectric effect appear (see Figure 1.2).
1.2.2. Polar solids
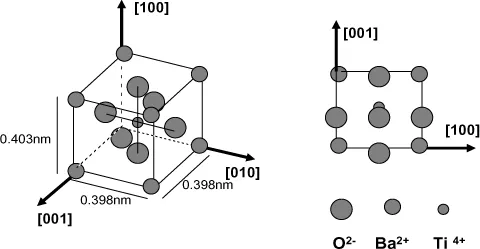
Polar solids are composed of polar molecules for which the centers of gravity of the positive and negative charges do not coincide (for example a water molecule); this is molecular polarization. This is the case for most molecular solids and ferroelectric solids, which present a spontaneous polarization. Figure 1.3 gives, for example, the structure of barium titanate, a typical case of a ferroelectric body (and therefore also piezoelectric).
1.2.3. Electronic polarization
Let us consider the spherical orbital of an electron. Under the influence of an external electric field
, the electrons are subject to a force -e
and the orbital gets distorted (see
Figure 1.4). Consequently, the centers of gravity of the positive and negative charges which were initially merged, no longer are: this is electric polarization, and this leads to the formation of an electrostatic dipole; therefore, a dipole moment internal to the atom is characterized by:
which opposes itself to the field
. α
elect is called the electronic polarisability. The polarization disappears if the field is removed.
1.2.4. Ionic polarization
In the case of ionic crystals, the average position of positive and negative ions changes under the influence of a field
. Suppose the ion is perfectly rigid from every angle. The action of the field will be to move it a quantity
with respect to a fixed mark centered in O; hence a variation of the polar moment:
This is the induced ionic polarization, proportional to the field (elastic distortions); where αion is the ionic polarisability.
The total dipole moment attached to the displacement of the ion and to the distortion of the electronic orbitals is, to a first approximation, the sum of [1.5] and [1.6], that is to say:
1.2.5. Orientation polarization
When we subject a polar molecule, carrier of a permanent dipole moment
, to an electric field
, its dipole tends to turn towards the direction of the field, which leads to a distortion of the molecule related to a torque: this is orientation polarization. This distortion is not instantaneous. There is the appearance of a hysteresis, on the one hand because the molecular forces tend to block its motion and, on the other hand, the thermal agitation will tend to disorient the molecules with respect to one another.
If
makes an angle θ with the direction of the field, the torque is:
The application of a field will have the effect on each molecule of producing a polar component in the direction of the field, whose first-order expression is:
α
or is called orientational polarisability. In general,
1.2.6. Interfacial or space-charge polarization
This type of polarization plays a part when the material possesses different phases or permittivity zones. Subje...