
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Introduction to Physical Polymer Science
About this book
An Updated Edition of the Classic Text
Polymers constitute the basis for the plastics, rubber, adhesives, fiber, and coating industries. The Fourth Edition of Introduction to Physical Polymer Science acknowledges the industrial success of polymers and the advancements made in the field while continuing to deliver the comprehensive introduction to polymer science that made its predecessors classic texts.
The Fourth Edition continues its coverage of amorphous and crystalline materials, glass transitions, rubber elasticity, and mechanical behavior, and offers updated discussions of polymer blends, composites, and interfaces, as well as such basics as molecular weight determination. Thus, interrelationships among molecular structure, morphology, and mechanical behavior of polymers continue to provide much of the value of the book.
Newly introduced topics include:
- Nanocomposites, including carbon nanotubes and exfoliated montmorillonite clays
- The structure, motions, and functions of DNA and proteins, as well as the interfaces of polymeric biomaterials with living organisms
- The glass transition behavior of nano-thin plastic films
In addition, new sections have been included on fire retardancy, friction and wear, optical tweezers, and more.
Introduction to Physical Polymer Science, Fourth Edition provides both an essential introduction to the field as well as an entry point to the latest research and developments in polymer science and engineering, making it an indispensable text for chemistry, chemical engineering, materials science and engineering, and polymer science and engineering students and professionals.
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
CHAPTER 1
INTRODUCTION TO POLYMER SCIENCE
1.1 FROM LITTLE MOLECULES TO BIG MOLECULES
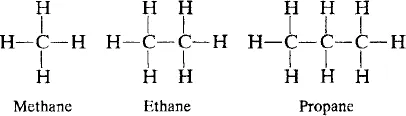
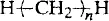
| Number of Carbons in Chain | State and Properties of Material | Applications |
| 1–4 | Simple gas | Bottled gas for cooking |
| 5–11 | Simple liquid | Gasoline |
| 9–16 | Medium-viscosity liquid | Kerosene |
| 16–25 | High-viscosity liquid | Oil and grease |
| 25–50 | Crystalline solid | Paraffin wax candles |
| 50–1000 | Semicrystalline solid | Milk carton adhesives and coatings |
| 1000–5000 | Tough plastic solid | Polyethylene bottles and containers |
| 3−6 × 105 | Fibers | Surgical gloves, bullet-proof vests |
Table of contents
- Cover
- Half Title page
- Title page
- Copyright page
- Dedication
- Preface to the Fourth Edition
- Preface to the First Edition
- Symbols and Definitions
- Chapter 1: Introduction to Polymer Science
- Chapter 2: Chain Structure and Configuration
- Chapter 3: Dilute Solution Thermodynamics, Molecular Weights, and Sizes
- Chapter 4: Concentrated Solutions, Phase Separation Behavior, and Diffusion
- Chapter 5: The Amorphous State
- Chapter 6: The Crystalline State
- Chapter 7: Polymers in the Liquid Crystalline State
- Chapter 8: Glass–Rubber Transition Behavior
- Chapter 9: Cross-Linked Polymers and Rubber Elasticity
- Chapter 10: Polymer Viscoelasticity and Rheology
- Chapter 11: Mechanical Behavior of Polymers
- Chapter 12: Polymer Surfaces and Interfaces
- Chapter 13: Multicomponent Polymeric Materials
- Chapter 14: Modern Polymer Topics
- Index