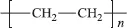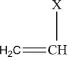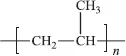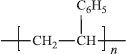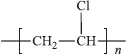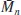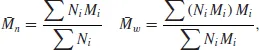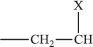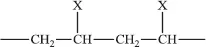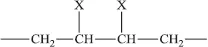![]()
1
Structure of Polymers
The mechanical properties that form the subject of this book are a consequence of the chemical composition of the polymer and also of its structure at the molecular and supermolecular levels. We shall therefore introduce a few elementary ideas concerning these aspects.
1.1 Chemical Composition
1.1.1 Polymerisation
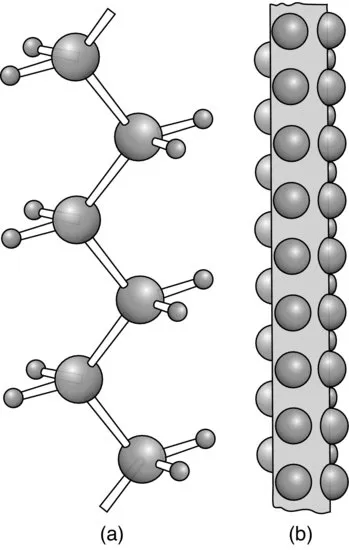
Linear polymers consist of long molecular chains of covalently bonded atoms, each chain being a repetition of much smaller chemical units. One of the simplest polymers is polyethylene, which is an addition polymer made by polymerising the monomer ethylene, CH2=CH2, to form the polymer.
Note that the double bond is removed during the polymerisation (Figure 1.1). The well-known vinyl polymers are made by polymerising compounds of the form.
where X represents a chemical group; examples are as follows:
Polypropylene
Polystyrene
and
poly(vinyl chloride)
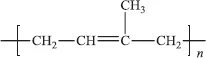
Natural rubber, polyisoprene, is a diene, and its repeat unit
contains a double bond.
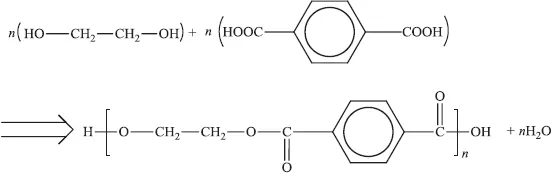
A condensation reaction is one in which two or more molecules combine into a larger molecule with or without the loss of a small molecule (such as water). One example is the formation of polyethylene terephthalate (the polyester used for Terylene and Dacron fibres and transparent films and bottles) from ethylene glycol and terephthalic acid:
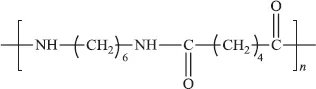
Another common condensation polymer is nylon 6,6.
1.1.2 Cross-Linking and Chain-Branching
Linear polymers can be joined by other chains at points along their length to make a cross-linked structure (Figure 1.2). Chemical cross-linking produces a thermosetting polymer, so called because the cross-linking agent is normally activated by heating, after which the material does not soften and melt when heated further, for example Bakelite and epoxy resins. A small amount of cross-linking through sulfur bonds is needed to give natural rubber its characteristic feature of rapid recovery from a large extension.
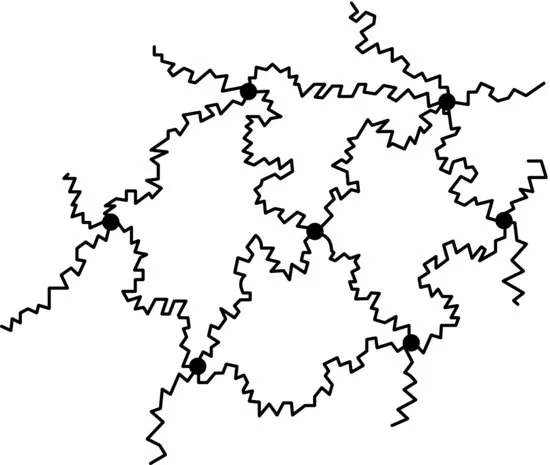
Very long molecules in linear polymers can entangle to form temporary physical cross-links, and we shall show later that a number of the characteristic properties of solid polymers are explicable in terms of the behaviour of a deformed network.
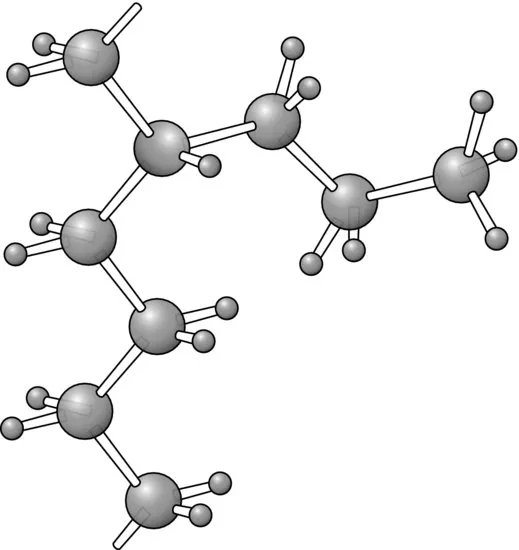
A less extreme complication is chain branching, where a secondary chain initiates from a point on the main chain, as is illustrated for polyethylene in Figure 1.3. Low-density polyethylene, as distinct from the high-density linear polyethylene shown in Figure 1.1, possesses on average one long branch per molecule and a larger number of small branches, mainly ethyl (—CH2—CH3) or butyl (—(CH2)3—CH3) side groups. The presence of these branch points leads to considerable differences in mechanical behaviour compared with linear polyethylene.
1.1.3 Average Molecular Mass and Molecular Mass Distribution
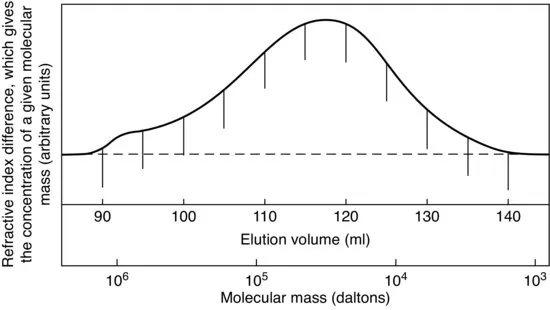
Each sample of a polymer contains molecular chains of varying lengths, that is of varying molecular mass (
Figure 1.4). The mass (length) distribution is of importance in determining the properties of the polymer, but until the advent of gel permeation chromatography [1,2] it could be determined only by tedious fractionation procedures. Most investigations therefore quoted different types of average molecular mass, the commonest being the number average
and the weight average
, defined as
where Ni is the number of molecules of molecular mass Mi, and Σ denotes summation over all i molecular masses.
The weight average molecular mass is always higher than the number average, as the former is strongly influenced by the relatively small number of very long (massive) molecules. The ratio of the two averages gives a general idea of the width of the molecular mass distribution.
Fundamental measurements of average molecular mass must be performed on solutions so dilute that intermolecular interactions can be ignored or compensated for. The commonest techniques are osmotic pressure for the number average and light scattering for the weight average. Both methods are rather lengthy, so in practice an average molecular mass was often deduced from viscosity measurements of either a dilute solution of the polymer (which relates to Mn) or a polymer melt (which relates to Mw). Each method yielded a different average value, which made it difficult to correlate specimens characterised by different groups of workers.
The molecular mass distribution is important in determining flow properties, and so may affect the mechanical properties of a solid polymer indirectly by influencing the final physical state. Direct correlations of molecular mass to viscoelastic behaviour and brittle strength have also been obtained.
1.1.4 Chemical and Steric Isomerism and Stereoregularity
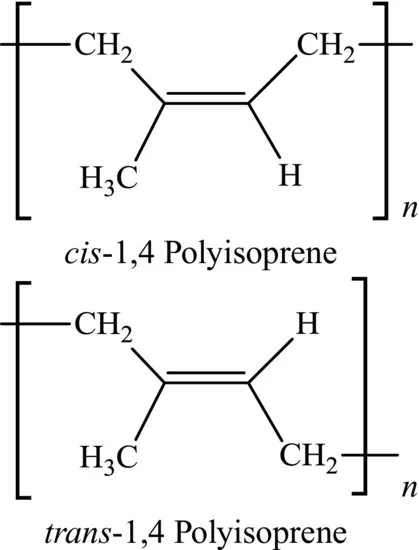
A further complication of the chemical structure of polymers lies in the possibility of different chemical isomeric forms within a repeat unit or between a series of repeat units. Natural rubber and gutta percha are chemically both polyisoprene, but the former is the cis form and the latter is the trans form (see Figure 1.5). The characteristic properties of rubber are a consequence of the loose packing of molecules (i.e. large free volume) that arises from its structure.
Vinyl monomer units
can be added to a growing chain either head-to-tail:
or head-to-head:
Head-to-tail substitution is usual, and only a small proportion of head-to-head linkages can produce a reduction in the tensile strength because of the loss of regularity.
Stereoregularity provides a more complex situation, which we will examine in terms of the simplest type of vinyl polymer (Figure 1.6) and for which we shall suppose that the polymer chain is a planar zigzag. Two very simple regular polymers can be constructed. In the first (Figure 1.6(a)) the substituent groups are all added in an identical manner to give an isotactic polymer. In the second regula...