
eBook - ePub
Medical Biochemistry
Human Metabolism in Health and Disease
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
About this book
Metabolism includes various pathways of chemical reactions; understanding these pathways leads to an improved knowledge of the causes, preventions, and cures for human diseases. Medical Biochemistry: Human Metabolism in Health and Disease provides a concise yet thorough explanation of human metabolism and its role in health and diseases. Focusing on the physiological context of human metabolism without extensive consideration of the mechanistic principles of underlying enzymology, the books serves as both a primary text and resource for students and professional in medical, dental, and allied health programs.
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Medical Biochemistry by Miriam D. Rosenthal,Robert H. Glew in PDF and/or ePUB format, as well as other popular books in Biological Sciences & Biochemistry. We have over one million books available in our catalogue for you to explore.
Information
CHAPTER 1
INTRODUCTION TO METABOLISM
1.1 INTRODUCTION
Intermediary metabolism is the name given to the sequences of biochemical reactions that degrade, synthesize, or interconvert small molecules inside living cells. Knowledge of the core metabolic pathways and their interrelations is critical to understanding both normal function and the metabolic basis of most human diseases. Rational interpretation and application of data from the clinical chemistry laboratory also requires a sound grasp of the major metabolic pathways. Furthermore, knowledge of key biochemical reactions in the two dozen or so core metabolic pathways in humans is essential for an understanding of the molecular basis of drug action, drug interactions, and the many genetic diseases that are caused by the absence of the activity of a particular protein or enzyme.
1.1.1 Metabolic Pathways
Metabolism occurs in small discrete steps, each of which is catalyzed by an enzyme. The term metabolic pathway refers to a particular set of reactions that carries out a certain function or functions. The pathway of gluconeogenesis or glucose synthesis, for example, operates mainly during a period of fasting, and its primary function is to maintain the concentration of glucose in the circulation at levels that are required by glucose-dependent tissues such as the brain and red blood cells. Another example of a metabolic pathway is the tricarboxylic acid (TCA) cycle, which oxidizes the two carbons of acetyl-coenzyme A (acetyl-CoA) to CO2 and water, thus completing the catabolism of carbohydrates, fats (fatty acids), and proteins (amino acids).
1.1.2 Metabolic Intermediates
Biochemical pathways are comprised of organic compounds called metabolic intermediates, all of which contain carbon, hydrogen, and oxygen. Some metabolic intermediates also contain nitrogen or sulfur. In most instances, these compounds themselves have no function. An exception would be a compound such as citric acid, which is both an intermediate in the TCA cycle and a key regulator of other pathways, including oxidation of glucose (glycolysis) and gluconeogenesis.
1.1.3 Homeostasis
Homeostasis refers to an organism’s tendency or drive to maintain the normalcy of its internal environment, including maintaining the concentration of nutrients and metabolites within relatively strict limits. A good example is glucose homeostasis. In the face of widely varying physiological conditions, such as fasting or exercise, both of which tend to lower blood glucose, or following the consumption of a carbohydrate meal that raises the blood glucose concentration, the human body activates hormonal mechanisms that operate to maintain blood glucose within rather narrow limits, 80 to 100 mg/dL (Fig. 1-1). Hypoglycemia (low blood glucose) stimulates the release of gluconeogenic hormones such as glucagon and hydrocortisone, which promote the breakdown of liver glycogen and the synthesis of glucose in the liver (gluconeogenesis), followed by the release of glucose into the blood. On the other hand, hyperglycemia (elevated blood glucose) stimulates the release of insulin, which promotes the uptake of glucose and its utilization, storage as glycogen, and conversion to fat.
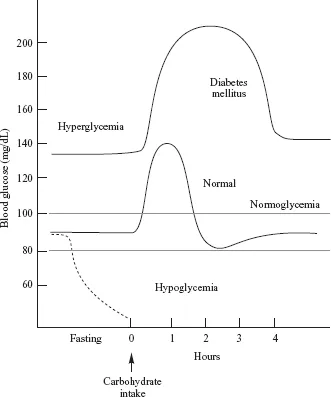
FIGURE 1-1 Changes that occur in the blood glucose concentration in a healthy adult, a person with type II diabetes mellitus, and a person experiencing fasting hypoglycemia. Following ingestion of a carbohydrate-containing meal, there are three features that distinguish the glucose vs. time curve for the person with type II diabetes relative to the healthy adult: (1) the initial blood glucose concentration is higher (approx. 135 vs. 90 mg/dL), (2) the rise in in the glucose level following the meal is greater; and (3) it takes longer for the glucose concentration to return to the fasting glucose level.
Maintenance of the blood calcium concentration between strict limits is another example of homeostasis. The normal total plasma calcium concentration is in the range 8.0 to 9.5 mg/dL. If the calcium concentration remains above the upper limit of normal for an extended period of time, calcium may deposit, with pathological consequences in soft tissues such as the heart and pancreas. Hypocalcemia (a.k.a. tetany) can result in muscle paralysis, convulsions, and even death; chronic hypocalcemia causes rickets in children and osteomalacia in adults. The body uses vitamin D and certain hormones (e.g., parathyroid hormone, calcitonin) to maintain calcium homeostasis.
1.2 WHAT DO METABOLIC PATHWAYS ACCOMPLISH?
1.2.1 Generation of Energy
The primary dietary fuels for human beings are carbohydrates and fats (triacylglycerols). The human body also obtains energy from dietary protein and—for some people—ethanol. Metabolism of these fuels generates energy, much of which is captured as the high-energy molecule adenosine triphosphate (ATP) (Fig. 1-2). The ATP can be used for biosynthetic processes (e.g., protein synthesis), muscle contraction, and active transport of ions and other solutes across membranes.
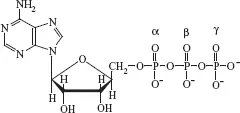
FIGURE 1-2 Structure of adenosine triphosphate.
1.2.2 Degradation or Catabolism of Organic Molecules
Catabolic pathways usually involve cleavage of C–O, C–N, or C–C bonds. Most intracellular catabolic pathways are oxidative and involve transfer of reducing equivalents (hydrogen atoms) to nicotinamide-adenine dinucleotide (NAD+) or flavine-adenine dinucleotide (FAD). The reducing equivalents in the resulting NADH or FADH2 can then be used in biosynthetic reactions or transferred to the mitochondrial electron-transport chain for generation of ATP.
1.2.2.1 Digestion.
Before dietary fuels can be absorbed into the body, they must be broken down into simpler molecules. Thus, starch is hydrolyzed to yield glucose, and proteins are hydrolyzed to their constituent amino acids.
1.2.2.2 Glycolysis.
Glycolysis is the oxidation of glucose into the three-carbon compound pyruvic acid.
1.2.2.3 Fatty Acid Oxidation.
The major route of fatty acid degradation is β-oxidation, which accomplishes stepwise two-carbon cleavage of fatty acids into acetyl-CoA.
1.2.2.4 Amino Acid Catabolism.
Breakdown of most of the 20 common amino acids is initiated by removal of the α-amino group of the amino acid via transamination. The resulting carbon skeletons are then further catabolized to generate energy or are used to synthesize other molecules (e.g., glucose, ketones). The nitrogen atoms of amino acids can...
Table of contents
- Cover
- Table of Contents
- Title
- Copyright
- PREFACE
- ACKNOWLEDGMENTS
- THE AUTHORS
- CHAPTER 1: INTRODUCTION TO METABOLISM
- CHAPTER 2: ENZYMES
- CHAPTER 3: DIGESTION AND ABSORPTION
- CHAPTER 4: GLYCOLYSIS
- CHAPTER 5: PYRUVATE DEHYDROGENASE AND THE TRICARBOXYLIC ACID CYCLE
- CHAPTER 6: ELECTRON TRANSPORT AND OXIDATIVE PHOSPHORYLATION
- CHAPTER 7: THE PENTOSE PHOSPHATE PATHWAY
- CHAPTER 8: GLYCOGEN
- CHAPTER 9: GLUCONEOGENESIS
- CHAPTER 10: FATTY ACID OXIDATION AND KETONES
- CHAPTER 11: FATTY ACID SYNTHESIS
- CHAPTER 12: TRIACYLGLYCEROL TRANSPORT AND METABOLISM
- CHAPTER 13: ETHANOL
- CHAPTER 14: PHOSPHOLIPIDS AND SPHINGOLIPIDS
- CHAPTER 15: EICOSANOIDS
- CHAPTER 16: GLYCOLIPIDS AND GLYCOPROTEINS
- CHAPTER 17: CHOLESTEROL SYNTHESIS AND TRANSPORT
- CHAPTER 18: STEROIDS AND BILE ACIDS
- CHAPTER 19: NITROGEN HOMEOSTASIS
- CHAPTER 20: AMINO ACIDS
- CHAPTER 21: SULFUR AMINO ACID METABOLISM
- CHAPTER 22: FOLATE AND VITAMIN B12 IN ONE-CARBON METABOLISM
- CHAPTER 23: PURINES AND PYRIMIDINES
- CHAPTER 24: HEME AND IRON
- CHAPTER 25: INTEGRATION OF METABOLISM
- INDEX
- End User License Agreement