
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
About this book
Medicinal Natural Products: A Biosynthetic Approach, Third Edition, provides a comprehensive and balanced introduction to natural products from a biosynthetic perspective, focussing on the metabolic sequences leading to various classes of natural products. The book builds upon fundamental chemical principles and guides the reader through a wealth of diverse natural metabolites with particular emphasis on those used in medicine.
There have been rapid advances in biosynthetic understanding over the past decade through enzymology, gene isolation and genetic engineering. Medicinal Natural Products has been extended and fully updated in this new edition to reflect and explain these developments and other advances in the field. It retains the user-friendly style and highly acclaimed features of previous editions:
- a comprehensive treatment of plant, microbial, and animal natural products in one volume
- extensive use of chemical schemes with annotated mechanistic explanations
- cross-referencing to emphasize links and similarities
- boxed topics giving further details of medicinal materials, covering sources, production methods, use as drugs, semi-synthetic derivatives and synthetic analogues, and modes of action
Medicinal Natural Products: A Biosynthetic Approach, Third Edition, is an invaluable textbook for students of pharmacy, pharmacognosy, medicinal chemistry, biochemistry and natural products chemistry.
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Medicinal Natural Products by Paul M. Dewick in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Industrial & Technical Chemistry. We have over one million books available in our catalogue for you to explore.
Information
1
ABOUT THIS BOOK, AND HOW TO USE IT
THE SUBJECT
This book has been written primarily for pharmacy students to provide a modern text to complement lecture courses dealing with pharmacognosy and the use of natural products in medicine. Nevertheless, it should be of value in other courses where the study of natural products is included, although the examples chosen are predominantly those possessing pharmacological activity.
For centuries, drugs were entirely of natural origin and composed of herbs, animal products, and inorganic materials. Early remedies may have combined these ingredients with witchcraft, mysticism, astrology, or religion, but it is certain that those treatments that were effective were subsequently recorded and documented, thus leading to the early Herbals. The science of pharmacognosy - the knowledge of drugs - grew from these records to provide a disciplined, scientific description of natural materials used in medicine. Herbs formed the bulk of these remedies. As chemical techniques improved, the active constituents were isolated from plants, were structurally characterized, and, in due course, many were synthesized in the laboratory. Sometimes, more active, better-tolerated drugs were produced by chemical modifications (semi-synthesis), or by total synthesis of analogues of the active principles.
Gradually, synthetic compounds superseded many of the old plant drugs, though certain plant-derived agents were never surpassed and remain as valued medicines to this day. Natural drugs derived from microorganisms have a much shorter history, and their major impact on medicine goes back only about 60 years to the introduction of the antibiotic penicillin. Microbially produced antibiotics now account for a very high proportion of the drugs commonly prescribed. There is currently a renewed interest in pharmacologically active natural products, be they from plants, microorganisms, or animals, terrestrial or marine, in the continued search for new drugs, particularly for disease states where our present range of drugs is less effective than we would wish. This is being reflected in a growing number of natural products or natural-product-inspired drugs entering medicine. Herbal remedies are also enjoying a revival as many sufferers turn away from modern drugs and embrace ‘complementary medicine’.
THE AIM
Many university pharmacy courses include a pharmacognosy component covering a study of plant-derived drugs; traditionally, this area of natural products has been taught separately from the microbially derived antibiotics, or the animal-related steroidal and prostanoid drugs. Such topics have usually formed part of a pharmaceutical chemistry course. The traditional boundaries may still remain, despite a general change in pharmacognosy teaching from a descriptive study to a phytochemical-based approach, a trend towards integrating pharmacognosy within pharmaceutical chemistry, and the general adoption of modular course structures. A chemistry-based teaching programme encompassing all types of natural products of medicinal importance, semi-synthetic derivatives, and synthetic analogues based on natural product templates is a logical development. This book provides a suitable text to complement such a programme, and attempts to break down the artificial divisions.
THE APPROACH
This book provides a groundwork in natural product chemistry/phytochemistry by considering biosynthesis -the metabolic sequences leading to various selected classes of natural products. This allows application of fundamental chemical principles and displays the relationships between the diverse structures encountered in nature, thus providing a rationale for natural products and replacing a descriptive approach with one based more on deductive reasoning. It also helps to transform complicated structures into a comprehensible combination of simpler fragments; natural product structures can be quite complex. Subdivision of the topics is predominantly via biosynthesis, not by class or activity, and this provides a logical sequence of structural types and avoids a catalogue effect. There is extensive use of chemical schemes and mechanism, with detailed mechanistic explanations being annotated to the schemes, as well as outline discussions in the text. Lots of cross-referencing is included to emphasize links and similarities; it is not necessary to follow these to understand the current material, but they are used to stress that the concept has been met before, or that other uses will be met in due course. As important classes of compounds or drugs are reached, more detailed information is then provided in the form of short separate monographs in boxes, which can be studied or omitted as required, in the latter case allowing the main theme to continue. The monograph information covers sources, production methods, principal components, drug use, mode of action, semi-synthetic derivatives, synthetic analogues, etc., as appropriate. Those materials currently employed as drugs, or being tested clinically, are emphasized in the monographs by the use of bold type.
THE TOPICS
A preliminary chapter is used to outline the main building blocks, the basic construction mechanisms employed in the biosynthesis of natural products, and how metabolic pathways are deduced. Most of the fundamental principles should be familiar and will have been met previously in courses dealing with the basics of organic chemistry and biochemistry. These principles are then seen in action as representative natural product structures are described in the following chapters. The topics selected are subdivided initially into areas of metabolism fed by the acetate, shikimate, mevalonate, and methylerythritol phosphate pathways. The remaining chapters then cover alkaloids, peptides and proteins, and carbohydrates. Not all classes of natural products can be covered, and the book is intended as an introductory text, not a comprehensive reference work.
The book tries to include a high proportion of those natural products currently used in medicine, the major drugs that are derived from natural materials by semi-synthesis, and those drugs which are structural analogues. Some of the compounds mentioned may have a significant biological activity which is of interest, but not medicinally useful. The book is also designed to be forward looking and gives information on possible leads to new drugs and materials in clinical trials.
THE FIGURES
A cursory glance through the book will show that a considerable portion of the content is in the form of chemical structures and schemes. The schemes and figures are used to provide maximum information as concisely as possible. The following guidelines should be appreciated:
- A figure may present a composite scheme derived from studies in more than one organism.
- Comments in italics provide an explanation in chemical terms for the biochemical reaction; detailed enzymic mechanisms are not usually considered.
- Schemes in separate frames show a mechanism for part of the sequence, the derivation of a substrate, or perhaps structurally related systems.
- Although enzymic reactions may be reversible, single rather than reversible arrows are used, unless the transformation is one that may be implicated in both directions, e.g. amino acid/keto acid transaminations.
- El, E2, etc., refer to enzymes catalysing the transformation, when known. Where no enzyme is indicated, the transformation may well have been determined by other methodology, e.g. isotope tracer studies. Speculative conversions may be included, but are clearly indicated.
- Enzyme names shown are the commonly accepted names; in general, only one name is given, even though alternative names may also be in current use.
- Proteins identified via the corresponding gene are often assigned a code name/number by researchers, and no systematic name has been proposed. This means that proteins carrying out the same transformation in different organisms may be assigned different codes.
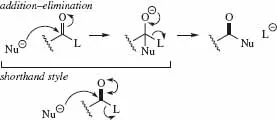
- Double-headed curly arrows are used to represent an addition-elimination mechanism as follows:
FURTHER READING
A selection of articles suitable for supplementary reading is provided at the end of each chapter. In general, these are not chosen from the primary literature, but are recent review articles covering broader aspects of the topic. They are also located in easily accessible journals rather than books, and have been chosen as the most student friendly. In certain cases, the most recent reviews available may be somewhat less up to date than the information covered in this book. All of the selected articles contain information considered appropriate to this book, e.g. reviews on ‘synthesis’ may contain sections on structural aspects, biosynthesis, or pharmacology.
WHAT TO STUDY
Coverage is fairly extensive to allow maximum flexibility for courses in different institutions, and not all of the material will be required for any one course. However, because of the many subdivisions and the highlighted keywords, it should be relatively easy to find and select the material appropriate for a particular course. On the other hand, the detail given in monographs is purposely limited to ensure students are provided with enough factual information, but are not faced with the need to assess whether or not the material is relevant. Even so, these monographs will undoubtedly contain data which exceed the scope of any individual course. It is thus necessary to apply selectivity, and portions of the book will be surplus to immediate requirements. The book is designed to be user friendly, suitable for modular courses and student-centred learning exercises, and a starting point for later project and dissertation work. The information presented is as up to date as possible; undoubtedly, new research will be published that modifies or even contradicts some of the statements made. The reader is asked always to be critical and to maintain a degree of flexibility when reading the scientific literature, and to appreciate that science is always changing.
WHAT TO LEARN
The primary aim of the book is not to rely just on factual information, but to impart an understanding of natural product structures and the way they are put together by living organisms. Rationalization based on mechanistic reasoning is paramount. The sequences themselves are not important, whilst the names of chemicals and the enzymes involved in the pathways are even less relevant and included only for information; it is the mechanistic explanations that are the essence. Students should concentrate on understanding the broad features of the sequences and absorb sufficient information to be able to predict how and why intermediates might be elaborated and transformed. The mechanistic explanations appended to the schemes should reinforce this approach. Anyone who commits to memory a sequence of reactions for examination purposes has missed the point. There is no alternative to memory for some of the material covered in the monographs, if it is required; wherever possible, information should be reduced ...
Table of contents
- Cover
- Title Page
- Copright
- Dedication
- 1: ABOUT THIS BOOK, AND HOW TO USE IT
- 2: SECONDARY METABOLISM: THE BUILDING BLOCKS AND CONSTRUCTION MECHANISMS
- 3: THE ACETATE PATHWAY: FATTY ACIDS AND POLYKETIDES
- 4: THE SHIKIMATE PATHWAY: AROMATIC AMINO ACIDS AND PHENYLPROPANOIDS
- 5: THE MEVALONATE AND METHYLERYTHRITOL PHOSPHATE PATHWAYS: TERPENOIDS AND STEROIDS
- 6: ALKALOIDS
- 7: PEPTIDES, PROTEINS, AND OTHER AMINO ACID DERIVATIVES
- 8: CARBOHYDRATES
- INDEX