
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Bioinorganic Medicinal Chemistry
About this book
This book gives a comprehensive overview about medicinal inorganic chemistry. Topics like targeting strategies, mechanism of action, Pt-based antitumor drugs, radiopharmaceuticals are covered in detail and offer the reader an in-depth overview about this important topic.
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
Chapter 1
Medicinal Inorganic Chemistry: State of the Art, New Trends, and a Vision of the Future
1.1 Introduction
Inorganic chemistry is an essential part of life. It is not just the chemistry of dead or inanimate things. It was probably even inorganic chemistry that started it all off. For example, iron sulfides may have been the energy sources for early forms of life [1]. There is currently emerging interest in the medicinal chemistry of the elements of the periodic table (Table 1.1).
Table 1.1 Some areas of medical interest in the elements of the periodic table. Entries are restricted to a few comments about each element and no attempt is made to be comprehensive. Elements thought to be essential for man are in italics.
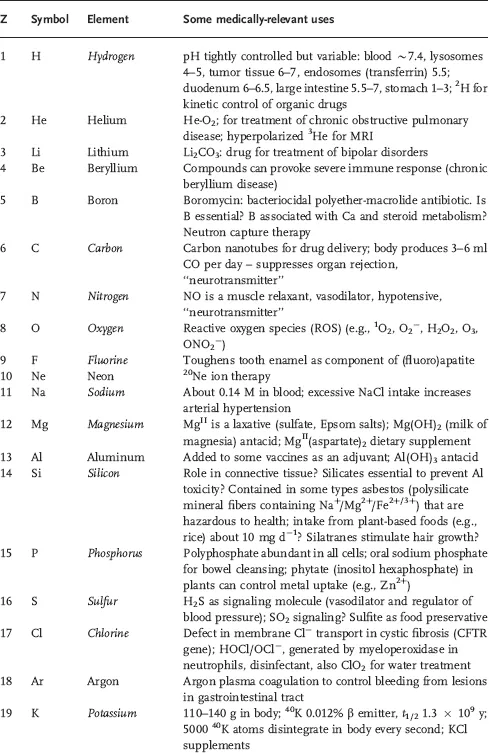
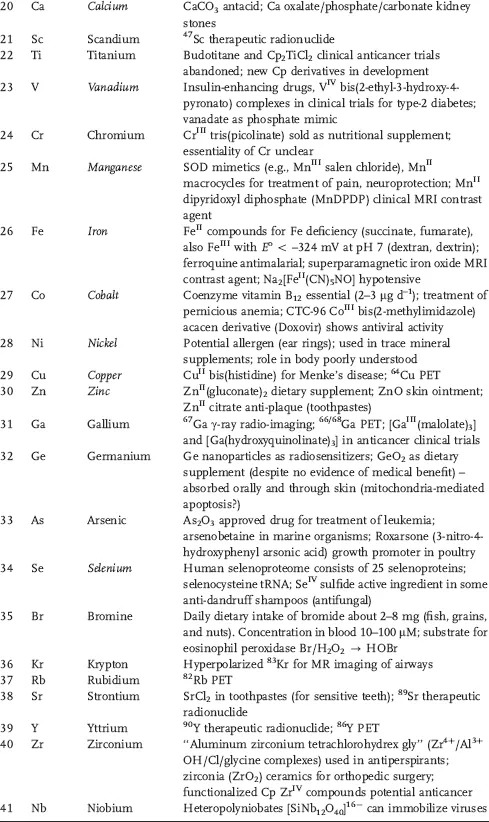

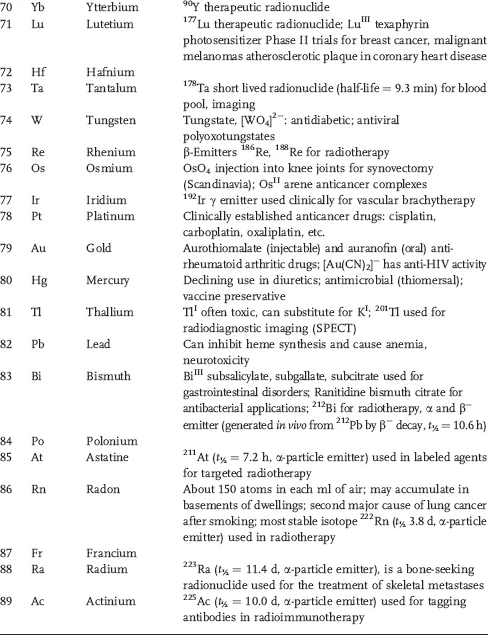
Currently 24 elements are thought to be essential for mammalian biochemistry (H, C, N, O, F, Na, Mg, Si, P, S, Cl, K, Ca, V, Mn, Fe, Co, Ni, Cu, Zn, Se, Mo, Sn, and I). However the biochemistry of some elements, particularly F, Si, V, Ni, and Sn is poorly understood. It has even been suggested that the biological requirement for Si is merely to protect against Al toxicity [2]. Interestingly, aluminum compounds are widely used as adjuvants in human and veterinary vaccines (helping and enhancing the pharmacological effect) although the chemical basis of the mechanism for this effect is not understood [3].
This situation with aluminum serves to illustrate the problem we face with inorganic medicines. They are often used on a mass scale, but with little rational basis and limited understanding of their molecular mechanism of action. Often we find this is because the methods and techniques available for the study of inorganic agents are either inadequate or are not fully exploited. In particular, determining the speciation of inorganic compounds under biological conditions remains a major challenge. Inorganic and metal compounds are often prodrugs that may not only be transformed on the way to target sites but also when attempts are made to extract the biologically active form from biological media.
This list of essential elements is probably not complete; for example, Cr and B may prove to be essential but the current evidence is unclear. Importantly, essentiality is not just about the element itself, but particular compounds of that element. For example, we need cobalt, but probably only in the form of the vitamin B12. Similarly, toxicity (often used as an argument against using metal compounds as drugs) is typically related not just to the metal itself but also to the ligands and to the type of complex.
Whilst a given metal does exhibit recurring features peculiar to itself (e.g., a preference for particular oxidation states and ligand geometries) the ligand environment can have a marked effect on the overall reactivity of the complex. Furthermore, the behavior of a metal complex is dependent on both its composition and the environment in which it finds itself. Predicting and controlling that behavior is one of the challenges for advancing the rational design of inorganic pharmaceuticals.
In this chapter we discuss transformations of metallodrugs by ligand exchange and/or redox processes, drawing on a wide range of examples. We attempt to relate these transformations to mechanisms of action with the aim of introducing rational design concepts to as many areas of medicinal inorganic chemistry as possible.
1.1.1 Metals in the Body: Essential Elements and Diseases of Metabolism
The homeostasis and control of metal ions in the body is an area of research in itself [4, 5]. Evolution has incorporated many metals into essential biological functions, using the variable oxidation states exhibited by the metal center (e.g., FeII/FeIII in heme) to control reversible binding of small molecules (e.g., O2) and implement structural changes. The ligand binding in a typical coordination M–L bond (50–150 kJ mol−1) is much weaker than covalent bonding (the energy of a single C-C bond is 300–400 kJ mol−1) [6]; for hemes and vitamin B12, for example, this allows much more flexibility in small molecule binding and dissociation (signaling) under biological conditions; the energies involved are much smaller. Other, even weaker, interactions such as hydrogen bonding (20–60 kJ mol−1) and van der Waal’s interactions (<50 kJ mol−1) are crucial for correct structure and functioning of biological systems. For metallodrugs such non-covalent interactions can play vital roles in target recognition.
A knowledge of the transport of metals and their complexes in vivo (particularly cell uptake and efflux) is important for understanding the metabolism of inorganic (and organic) drugs, and also for understanding the nature of diseases caused by erroneous metal transport.
1.1.2 Metals as Therapeutic Agents
Medicinal inorganic chemistry is a relatively young, interdisciplinary research area that has grown primarily due to the success of cisplatin, a Pt-based anticancer drug developed in the late 1960s. In addition to metal-centered therapies, metals may also be used to enhance the efficacy of organic drugs (such as the cyclams, e.g., AMD3100) and as small-molecule delivery vehicles (e.g., for NO, CO).
Organic compounds used in medicine may be activated by metal ions or metalloenzymes, and others can have a direct or indirect effect on metal ion metabolism. Since many organic drugs follow conventional design rules (e.g., Lipinski’s rule of 5), they typically incorporate groups with the ability to act as electron donors (H-bond acceptors), endowing them with potential metal-binding sites. This bioinorganic reactivity needs to be considered when new organic drugs are designed. The rational design of metal-based drugs is a relatively new concept, bolstered by improvements in characterization and imaging techniques. In general a metal complex that is administered is likely to be a “prodrug” that undergoes a transformation in vivo before reaching its target site. Such transformations can include reduction or oxidation of the metal ion, ligand substitution, or reactions of the ligands at sites remote from the metal. Elucidating the precise mechanisms of action of these new drugs is perhaps the most challenging and complicated aspect of the research; it requires drawing together knowledge concerning the reactivity of the metal complex (outlined in Figure 1.1 and Table 1.2) to control features such as biochemical stability, coupled with an appreciation of the biochemical pathways governing cell uptake, metabolism, and excretion. By appropriate choice of the ligands and metal oxidation state, it is possible to control the thermodynamic and kinetic properties of metal complexes and to attempt to control their biological activity [7].
Figure 1.1 Some features of metal coordination complexes that can play a role in biological activity. Control of these characteristics is important in rational design.
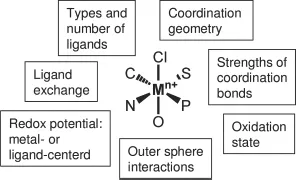
Table 1.2 Features of metals and metal complexes that can be used in the design of therapeutic and diagnostic agents.
| Feature | Comments (examples) |
| Coordination number | Full range 2–10; transition metals typically 4–6, can be more variable for main group metals (e.g., Bi)... |
Table of contents
- Cover
- Contents
- Title
- Copyright
- List of Contributors
- Chapter 1 : Medicinal Inorganic Chemistry: State of the Art, New Trends, and a Vision of the Future
- Chapter 2 : Targeting Strategies for Metal-Based Therapeutics
- Chapter 3 : Current Status and Mechanism of Action of Platinum-Based Anticancer Drugs
- Chapter 4 : New Trends and Future Developments of Platinum-Based Antitumor Drugs
- Chapter 5 : Ruthenium and Other Non-platinum Anticancer Compounds
- Chapter 6 : The Challenge of Establishing Reliable Screening Tests for Selecting Anticancer Metal Compounds
- Chapter 7 : Gold-Based Therapeutic Agents: A New Perspective
- Chapter 8 : MRI Contrast Agents: State of the Art and New Trends
- Chapter 9 : Metal-Based Radiopharmaceuticals
- Chapter 10 : Boron and Gadolinium in the Neutron Capture Therapy of Cancer
- Chapter 11 : Essential Metal Related Metabolic Disorders
- Chapter 12 : Metal Compounds as Enzyme Inhibitors
- Chapter 13 : Biomedical Applications of Metal-Containing Luminophores
- Index
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Bioinorganic Medicinal Chemistry by Enzo Alessio in PDF and/or ePUB format, as well as other popular books in Ciencias físicas & Química industrial y técnica. We have over one million books available in our catalogue for you to explore.