![]()
Chapter 1
Introduction
Mihai D. Niculescu1 and Paul Haggarty2
1Department of Nutrition and Nutrition Research Institute, University of North Carolina at Chapel Hill, Kannapolis, NC, USA
2Nutrition & Epigenetics Group, Rowett Institute of Nutrition & Health, University of Aberdeen, Aberdeen, Scotland, UK
1.1. ADAPTATION, AN EVOLVING CONCEPT
The concept of adaptation was, and still is, considered one of the most important principles of biology. Related to the idea of transformation—epigenesis in Aristotle's words—adaptation exists as a means to better cope with environmental changes, whether on a long or a short term. In 1809, Jean-Baptiste Lamarck published his Philosophie zoologique ou exposition des considérations relatives à l’histoire naturelle des animaux, in which he argued that characteristics acquired during the life of an individual (because of exposure to various environmental influences) can be transmitted to the young during reproduction (soft inheritance). Since its publication, and until recently, Lamarck's theory of soft inheritance has been largely disregarded, while Darwin's theory of evolution became predominant in modern biology (Handel and Ramagopalan, 2010).
The resurrection of the soft inheritance concept manifested only recently, when it became obvious that environmental influences could trigger metabolic and phenotypic changes that could be transmitted to subsequent generations, even when such exposures were present only during the life of the first generation (Chmurzynska, 2010). Animal studies indicated that acquired characteristics could be inherited, and that practically any type of environmental changes might initiate such events (maternal nutrition, gestational exposure to endocrine disrupting chemicals, ionizing radiation, etc.) (Youngson and Whitelaw, 2008.
Potential mechanisms for the propagation of such influences from the parent to offspring are many, including poor maternal health (inducing similar phenotypes in the next generation), behavioral interactions (perpetuation of the same phenotype by either similar behavior or endocrine changes that are perpetuated between generations), postfertilization transfer of viruses or toxins, and epigenetic mechanisms (Youngson and Whitelaw, 2008). This book focuses, among other epigenetic mechanisms responsible for shaping gene–nutrient interactions, on epigenetic inheritance, which consists of the transmission of parental epigenetic patterns across the generations, and how nutrition may impact on this.
There are many definitions of epigenetics. Coined by Waddington in 1942 (Waddington, 1942), today epigenetics refers to the study of heritable patterns of gene expression that are not caused by changes in DNA sequence. The heritability of gene expression patterns refers to both cell division and transgenerational inheritance.
The epigenetic interaction between our genes and the environment allows a time-efficient process of adaptation that starts with the embryonic and fetal stages of development.
1.2. EPIGENETIC MECHANISMS AND THEIR ROLES
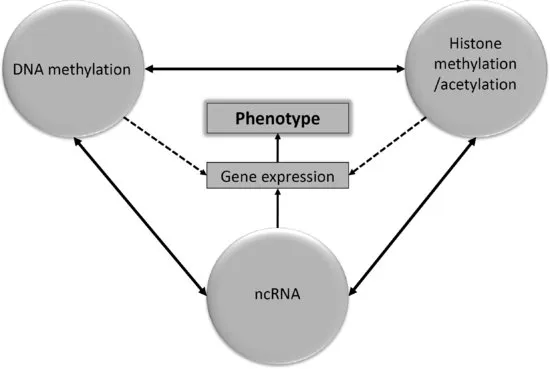
The epigenetic mechanisms consist of complex interactions between DNA and nuclear proteins (mainly histones), which define the pattern of gene expression in a given cell. These DNA–histone interactions, as well as gene expression, are also influenced by small, noncoding RNA (ncRNA) molecules, which further modulate the pattern of gene expression that defines a specific cellular phenotype (Figure 1.1).
DNA methylation was first described as a natural chemical modification in 1950 (Wyatt, 1950), but its relationship with DNA activation remained unclear until 1971 when de Waard demonstrated that the biological activity of DNA was modulated by its methylation in vitro (de Waard, 1971). Soon it became clear that DNA methylation was a dynamic process that varied across different phases of the cell cycle, and that the amount of DNA methylation might be related to the active and inactive states of chromatin (Comings, 1972). The role of DNA methylation in regulating gene expression was clearly hypothesized by Venner and Reinert in 1973 (Venner and Reinert, 1973). The idea that DNA methylation could profoundly influence gene expression led to the hypothesis that the inactivation of chromosome X was epigenetic (Riggs, 1975), and the opposite relationship between DNA methylation and gene expression was established later (Christman et al., 1977). Since then, our understanding of the role that DNA methylation plays in gene expression and phenotype inheritance has increased exponentially.
Interestingly, the role of histone modifications was recognized earlier than that of DNA methylation. In 1964, both methylation and acetylation of histones were reported to have a role in RNA synthesis (Allfrey et al., 1964). However, because such histone modifications proved to be much more complex than DNA methylation, the progress was slower. Only within the last 10–15 years, the complex study of histone modifications became available due to the technological advances in detection of methylation/acetylation of various amino acids in these proteins.
The discovery of the role of ncRNA in modulating DNA methylation and histone acetylation/methylation is much recent. Chromatin remodeling by ncRNA plays an important role in gene silencing and holds the premise that epigenetic therapy is possible for targeting specific genes, in specific tissues, using targeted incorporation of silencing exogenous ncRNA (Malecova and Morris, 2010).
1.3. NUTRITION, EPIGENETICS, AND HEALTH
It is becoming clear that epigenetic regulation is a fundamental process that impinges on many areas of human biology relevant to nutrition and health. The challenge is to identify the causal connections between epigenetics and health and elucidate the way in which diet might influence these processes. Such work offers the hope of developing early epigenetic markers of disease, improving dietary and lifestyle advice to maintain health into old age and improving treatments through the elucidation of mechanisms.
Much is already known about epigenetic changes in cancer. A common observation in cancer is epigenetic change consisting of altered methylation of DNA and the histones associated with DNA. These changes occur early in the development of the disease and the pattern of methylation correlates with cancer stage (Szyf et al., 2004). More interesting is the possibility that widespread epigenetic change in normal cells may actually be causal in the transition to cancer and that nutrition may influence this process. The evidence in relation to this is discussed in the subsequent chapters. There is also growing interest in the role of epigenetic processes in the other major diseases such as cardiovascular disease and diabetes though much less is known about the role of epigenetics in these conditions. Epigenetics is also of growing interest in relation to normal and aberrant biological function in fields as diverse as cognition and reproduction. These are also discussed.
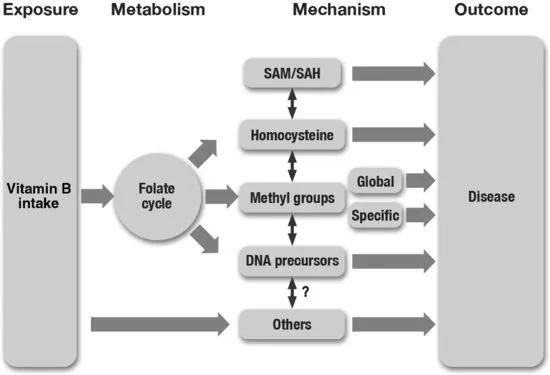
One of the problems encountered when attempting to establish causality between nutrition, epigenetics, and health is covariance between the markers of many of the candidate hypotheses (Figure 1.2). This is particularly problematic in human studies where much of the evidence arises from observational designs. Take the example of human vascular disease. A large number of observational studies have identified an inverse relationship between the dietary intake of folate and other B vitamins, and incidence of vascular disease (Rimm et al., 1998; He et al., 2004; Tavani et al., 2004). Further evidence for the role of B vitamins is provided by genetic association studies linking polymorphisms in the genes involved in B vitamin metabolism to the risk of coronary heart disease, cardiovascular disease, and stroke (Klerk et al., 2002; Wald et al., 2002; Casas et al., 2004). However, the mechanism by which B vitamin status and related genotype might influence cardiovascular health remains obscure. Until recently, the main hypothesis was that B vitamin status influenced vascular health via an effect on circulating homocysteine levels. However, despite a great deal of research in this field, a causal role for homocysteine has yet to be established (Brattstrom and Wilcken, 2000) and there is evidence that homocysteine may not be the causal link (Moat, 2004a, 2004b; Durga et al., 2005). Because both homocysteine and methyl groups are produced by the activity of the same folate/methylation pathway, it is possible that homocysteine may be acting as a proxy for health effects mediated by methylation and that the observational link between B vitamin status and health is mediated by an epigenetic mechanism.
The continuing uncertainty over B vitamin-related causal mechanisms partly arises because of the covariance of metabolite concentrations and biological functions, which depend on the folate/methylation cycle. Blood folate, homocysteine, and MTHFR C677T genotype all covary, and this linkage also appears to extend to global DNA methylation (Friso et al., 2005) with the level of methylation being correlated with plasma homocysteine (Castro et al., 2003). Furthermore, homocysteine is often correlated with the concentration of other folate/methylation cycle intermediates such as S-adenosyl-methionine and S-adenosyl-homocysteine (SAH) (James et al., 2002). The difficulty of interpreting this information is highlighted by the proposal that the often-reported association between homocysteine and disease may arise because homocysteine is acting as a proxy for a causal effect operating through DNA methylation since SAH is a potent inhibitor of the DNA methyltransferases and it changes in parallel with homocysteine concentration (James et al., 2002). Alcohol is another factor known to influence this cycle, disease risk, and methylation status. Animal models of chronic alcohol exposure result in altered DNA (Garro et al., 1991; Choi et al., 1999) and histone (Kim and Shukla, 2005) methylation. DNA methylation has also been shown to vary with alcohol exposure in humans (Bonsch et al., 2004, 2005). There is some evidence for epigenetic mediation of vascular disease. Direct evidence comes from studies in animals. Altered global DNA methylation has been observed in mouse and rabbit atherosclerotic lesions (Hiltunen et al., 2002). Studies in an atherogenic mouse model have shown that altered DNA methylation precedes the development of atherosclerosis (Lund et al., 2004) while proliferation of vascular smooth muscle cells is thought to be influenced by changes in DNA methylation (Post et al., 1999; Ying et al., 2000). Atherosclerosis in humans is also associated with altered DNA methylation compared with healthy controls (Castro, 2003). There are other ways in which nutrition can influence disease risk through epigenetic mechanisms, and these are covered in subsequent chapters, but the covariance often observed between multiple candidate processes illustrates the difficulty in establishing causal links between nutrition, epigenetics, and human health (Haggarty 2007).
1.4. CONCLUSION
The process of epigenetic marking is fundamental to homeostasis and the healthy functioning of cells. Modulation of epigenetic status c...