
Introduction to Quantum Mechanics with Applications to Chemistry
- 496 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Introduction to Quantum Mechanics with Applications to Chemistry
About this book
When this classic text was first published in 1935, it fulfilled the goal of its authors "to produce a textbook of practical quantum mechanics for the chemist, the experimental physicist, and the beginning student of theoretical physics." Although many who are teachers today once worked with the book as students, the text is still as valuable for the same undergraduate audience.
Two-time Nobel Prize winner Linus Pauling, Research Professor at the Linus Pauling Institute of Science and Medicine, Palo Alto, California, and E. Bright Wilson, Jr., Professor Emeritus of Chemistry at Harvard University, provide a readily understandable study of "wave mechanics," discussing the Schrodinger wave equation and the problems which can be solved with it. Extensive knowledge of mathematics is not required, although the student must have a grasp of elementary mathematics through the calculus. Pauling and Wilson begin with a survey of classical mechanics, including Newton's equations of motion in the Lagrangian form, and then move on to the "old" quantum theory, developed through the work of Planck, Einstein and Bohr. This analysis leads to the heart of the book ― an explanation of quantum mechanics which, as Schrodinger formulated it, "involves the renunciation of the hope of describing in exact detail the behavior of a system." Physics had created a new realm in which classical, Newtonian certainties were replaced by probabilities ― a change which Heisenberg's uncertainty principle (described in this book) subsequently reinforced.
With clarity and precision, the authors guide the student from topic to topic, covering such subjects as the wave functions for the hydrogen atom, perturbation theory, the Pauli exclusion principle, the structure of simple and complex molecules, Van der Waals forces, and systems in thermodynamic equilibrium. To insure that the student can follow the mathematical derivations, Pauling and Wilson avoid the "temptation to condense the various discussions into shorter and perhaps more elegant forms" appropriate for a more advanced audience. Introduction to Quantum Mechanics is a perfect vehicle for demonstrating the practical application of quantum mechanics to a broad spectrum of chemical and physical problems.
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
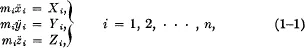

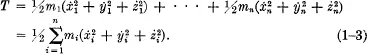
Table of contents
- Cover
- Title Page
- Copyright Page
- Preface
- Contents
- Chapter I: Survey of Classical Mechanics
- Chapter II: The Old Quantum Theory
- Chapter III: The Schrödinger Wave Equation with the Harmonic Oscillator as an Example
- Chapter IV: The Wave Equation for a System of Point Particles in Three Dimensions
- Chapter V: The Hydrogen Atom
- Chapter VI: Perturbation Theory
- Chapter VII: The Variation Method and other Approximate Methods
- Chapter VIII: The Spinning Electron and the Pauli Exclusion Principle, with a Discussion of the Helium Atom
- Chapter IX: Many-Electron Atoms
- Chapter X: The Rotation and Vibration of Molecules
- Chapter XI: Perturbation Theory Involving the Time, the Emission and Absorption of Radiation, and the Resonance Phenomenon
- Chapter XII: The Structure of Simple Molecules
- Chapter XIII: The Structure of Complex Molecules
- Chapter XIV: Miscellaneous Applications of Quantum Mechanics
- Chapter XV: General Theory of Quantum Mechanics
- Appendix I: Values of Physical Constants
- Appendix II: Proof that the Orbit of a Particle Moving in a Central Field Lies in a Plane
- Appendix III: Proof of Orthogonality of Wave Functions Corresponding to Different Energy Levels
- Appendix IV: Orthogonal Curvilinear Coordinate Systems
- Appendix V: The Evaluation of the Mutual Electrostatic Energy of Two Spherically Symmetrical Distributions of Electricity with Exponential Density Functions
- Appendix VI: Normalization of the Associated Legendre Functions
- Appendix VII: Normalization of the Associated Laguerre Functions
- Appendix VIII: The Greek Alphabet
- Index