![]()
CHAPTER 1
Introduction to Bijels
P. S. Clegg* and J. H. J. Thijssen
SUPA School of Physics & Astronomy, The University of Edinburgh, Peter Guthrie Tait Road, Edinburgh, EH9 3FD, UK,
*E-mail:
[email protected] The bijel is a soft composite material with unusual characteristics that make it suitable, for example, for catalysis, filtration and electrode/electrolyte applications. The name is an acronym for bicontinuous interfacially jammed emulsion gel; it is a member of the family of emulsions with interfaces stabilized by colloidal particles. Conventional particle-stabilized (Pickering–Ramsden) emulsions have a dispersed liquid phase in the form of droplets and a continuous liquid phase that surrounds them. A bijel has two continuous liquid phases that are mutually entangled in a tortuous pattern, with a particle-stabilized interface between. Bijels were originally conceived in silico and conventionally fabricated by arresting the spinodal pattern of phase-separating liquids. The purpose of this chapter is to present the bijel concept as initially developed. This provides the foundation for the more recent innovations covered in subsequent chapters. We begin by putting the bijel idea in the context of the liquid-crystal research that immediately preceded it. We then explain the practicalities of making bijels, the processing route and the characteristics of the final samples. We briefly mention related research on freeze-casting porous ceramics, which occurred in parallel and is another example of using a phase transition in a host solvent to structure colloidal particles. Finally, we highlight some very recent research on carboxysomes, where self-organization driven by phase transition kinetics is being used in a very different context.
1.1 Background
Self-assembly of colloidal components is an important means of building materials and objects from the micrometre scale upwards. It is often achieved by controlling the chemical bonds formed between the components. 1 Most spectacularly, this has been implemented via the use of carefully chosen DNA segments that are attached to solid particle or droplet surfaces. 2 In this case, the DNA can be directly bonded to complementary strands or via the use of linker molecules. 3 The topic of this chapter is a profoundly different approach: organizing particles by using the phase transition kinetics of the host solvent. This has two advantages: firstly, the pattern of organization comes from the solvent and not from the building blocks. Hence, the same approach will work for a broad variety of starting materials. Secondly, because the approach relies on the host solvent being driven out of thermodynamic equilibrium, it is history-dependent. This makes it possible to tune the formation route via changes to parameters such as composition, quench depth, quench rate, etc. Organizing particles, by using the phase transition kinetics of the host solvent, makes it possible to achieve the same result using different particles and to controllably achieve different results using the same particles. This is a versatile approach. Towards the end of the chapter, we will see how organization via phase separation is being used to explain carboxysome formation in cyanobacteria. In this case, both specific interactions between components and generic phase separation are important.
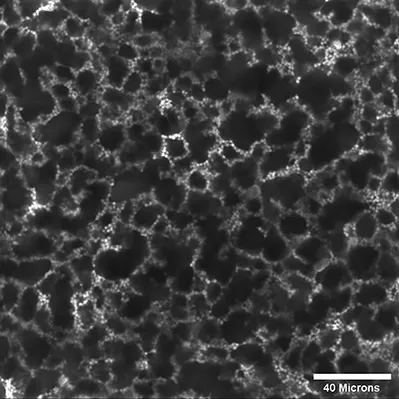
The bijel forms when liquids that demix via spinodal decomposition are used to assemble particles. This research rose to prominence with the publication of computer simulations 4 in 2005 and their subsequent experimental realization in 2007. 5 However, the idea of structuring liquids using complex host solvents had been explored previously, for example, by working with composites of liquid crystals and colloidal particles. The soft matter group at the University of Edinburgh, led in turn by Pusey, Cates and then Poon, had spent many years studying dispersions of sterically stabilized poly(methyl methacrylate) colloidal particles (PMMA): firstly, as model hard spheres and, secondly, with non-adsorbing polymers as a model of colloidal depletion interactions. Meeker, Poon and collaborators went on to examine the behaviour of PMMA particles in a highly unconventional solvent. 6 Meeker et al. used a low-molecular-weight liquid crystal 4-n-pentyl-4′-cyanobiphenyl (5CB) that exhibits an isotropic to nematic phase transition at T IN ≈ 35 °C. The experiments using ∼5 vol% of particles revealed that a particle network formed during the temperature quench and that this conveyed great mechanical strength to the liquid crystal (Figure 1.1). Subsequent research explored the details of the network, its properties and its formation. 7
Figure 1.1 Network formation in a sample of 5CB with φ w = 0.05 of σ = 0.7 µm PMMA particles cooled through the isotropic–nematic transition at T IN down to T = 15 °C. After five minutes at this temperature, the samples were removed from the temperature stage. The resulting network structure was studied via fluorescence confocal microscopy using a Nikon microscope and the 488 nm line of an Ar-ion laser.
The research on 5CB–PMMA composites set the scene for the bijel project. The phase transition kinetics of the host solvent and the relationship between the particles and the ordered and disordered phases play crucial roles in structure self-organization. In the isotropic phase, molecular orientation is random, whereas, in the nematic phase, the molecules have a preference for alignment along a specific axis. On cooling, nuclei of ordered nematic appear and begin to confine the PMMA particles in the residual isotropic phase due to the elastic energy penalty of leaving a PMMA particle in a well-ordered nematic region. The nuclei progressively coarsen, which ultimately confines the particles in high volume fraction strands with disordered liquid crystal in the interstices.
At a conceptual level, the isotropic–nematic transition is characterized by a non-conserved order parameter. Once the phase transition has gone to completion, none of the high temperature disordered (isotropic) phase remains. Hence, particle network formation is what occurs as the particles are excluded from the ordered regions. The bijel project was then launched as the result of Cates (then also at the University of Edinburgh) asking the question: what would happen to particles dispersed in the disordered phase of a system characterized by a conserved order parameter? He then articulated some of the ideas subsequently researched and described in this book as claims in a patent filed in 2006. 8 At a transition with a conserved order parameter the aspects of the system that will go on to form the ordered phase already exist in the disordered phase. Phase-separating liquids are an example of just such a system. 4
Whether the order parameter is conserved or non-conserved has profound implications for the dynamics of the phase transition and this, in turn, is important when the transition is being employed to organize dispersed particles. When an order parameter is conserved through a phase transition, components have to move around within the sample for the order to develop. 9 In phase-separating liquids, this occurs via diffusion at an early stage and is then driven by Laplace pressure gradients during late-stage coarsening. 10 As a bijel begins to form, this is crucial because the incipient arrangement of particles must allow material to diffuse across the interface. Likewise, at a later stage during bijel formation the jammed particles must finally prevent large-scale flow parallel to the interface. By contrast, in a system characterized by a non-conserved order parameter, the phase transition dynamics do not involve components moving. However, this does not mean that nothing moves during the formation of, for example, liquid-crystal particle composites. As regions of the sample order, and as the order grows, the particulate component will be swept along by the ordering front. A crucial characteristic in this case is whether the particles have too much inertia to be moved by this ordering front. 11
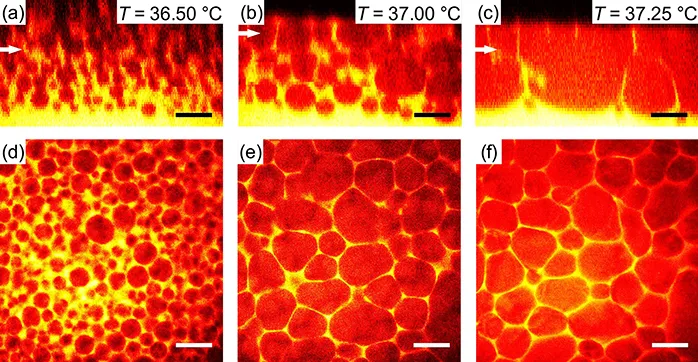
As an aside, it is important to note that it is also possible to create cellular networks, such as those observed in 5CB–PMMA composites, in partially miscible binary fluid systems (see Figure 1.2), i.e., via a transition with a conserved order parameter. 12 In this case, emulsions are created via nucleation and growth following an off-critical quench of a dispersion of silica particles in an oil/alcohol mixture. Droplets of the minority phase form and particles are both swept up onto the liquid interface and trapped between the droplets. Here, the tuning of the particle wettability is not crucial and the emulsion has long-term stability from a combination of interfacial particles and a surplus population in the continuous phase. Network formation then occurs on re-warming the system towards the mixed phase. Due to creaming of the droplets, a change in (local) sample composition has occurred, with much of the continuous phase separated at the base of the sample. Hence, during warming the droplets expand, largely due to coalescence, and the particles become increasingly concentrated in the space between the droplets. A faceted network is found to form (see Figure 1.2).A similar change in sample composition, leading to network formation, can also be achieved via evaporation of one of the liquid phases from a particle-stabilized emulsion. 13
Figure 1.2 Three-dimensional structure from two-photon fluorescence images of the formation of a cellular network by step-wise heating of a creamed hexane–methanol/silica emulsion: (a,c,e) vertical xz slices and (b,d,f) horizontal xy slices; the latter were taken at the heights indicated by white arrows in panels a,c,e. Sample temperature was constant for ≥3 min prior to imaging; heating rates were ∼0.1 °C min−1. Scale bars: 200 µm. Reproduced from ref. 12 with permission from the Royal Society of Chemistry.
Returning to bijels, there are important experimental results that pre-date the fabrication of bijels from low-molecular-weight liquids. In 1994,Gubbels et al. used carbon black particles to stabilize a blend of polyethylene and polystyrene polymers. 14 They needed the carbon black particles to percolate through the sample to give good electrical conductivity. Unsurprisingly, the best blend stabilization and conductivity properties were achieved with the particles trapped at the interface between two tortuous polymer domains. In 2005, Composto and his team prepared two-dimensional bijels in thin polymer films (see Chapters 3 and 4). This geometry allowed them to explore the relationship between the morphology and the wettability of the external surfaces of the samples. While both of these projects resulted in the formation of polymer blend-based composites, the work on three-dimensional sam...