
- 336 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Phytoestrogens In Functional Foods
About this book
Polyphenol phytoestrogens - bioactives found in specific foods and beverages - impart antioxidant, phytoestrogenic, antiproliferative, and enzyme modulating activities within the human metabolic system. It is believed that these compounds protect against several forms of cancer, cardiovascular and neurodegenerative diseases, osteoporosis, and menop
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Phytoestrogens In Functional Foods by Fatih Yildiz in PDF and/or ePUB format, as well as other popular books in Technology & Engineering & Food Science. We have over one million books available in our catalogue for you to explore.
Information
Part I
Production
1
Introduction to Phytoestrogens
E. Gültekin and Fatih Yildiz
CONTENTS
- 1.1 Phytoestrogens
- 1.1.1 Classification of Phytoestrogens
- 1.1.2 Structure-Function Similarities of Phytoestrogens
- 1.1.3 Estrogenic Properties of Phytoestrogens
- 1.1.4 Relative Estrogenic Activity of Phytoestrogens
- 1.2 Isoflavones
- 1.2.1 Classes of Isoflavones
- 1.2.2 Physical and Chemical Properties of Isoflavones
- 1.2.2.1 Water Solubility
- 1.2.2.2 Chemical StabilityChemical StabilityChemical Stability
- 1.2.3 Absorption, Distribution, Metabolism, and Excretion of Isoflavones
- 1.3 Analysis of Phytoestrogens
- 1.3.1 Isolation of Phytoestrogens
- 1.3.2 Analytical Methods
- 1.4 Reported Phytoestrogen Content of Foods
- 1.5 The Future Trends of Phytoestrogen Research
- References
1.1 Phytoestrogens
Phytoestrogens are naturally occurring chemicals of plant origin that have the ability to cause estrogenic and/or antiestrogenic effects due to their structural similarities to the human hormone estradiol (17β-estradiol).1
1.1.1 Classification of Phytoestrogens
The majority of phytoestrogens belong to a large group of substituted phenolic compounds known as flavonoids. There are several groups of flavonoids with estrogenic properties. Of these, coumestans and isoflavones possess the greatest estrogenic activity.2 A class of prenylated flavonoids with estrogenic activities intermediate to those of the coumestans and isoflavones has recently been identified.3 Lignans, a class of nonflavonoid phytoestrogens, have also been shown to exert estrogenic effects.4 The relationship between these types of phytoestrogens and the names of the compounds most commonly found in food from these four groups is summarized in Figure 1.1.
1.1.2 Structure-Function Similarities of Phytoestrogens
Similarity of phytoestrogens to estrogens at the molecular level provides them the ability to mildly mimic and in some cases act as an antagonist to estrogen.5 Common features of phytoestrogens and estradiol are listed in Table 1.1.6
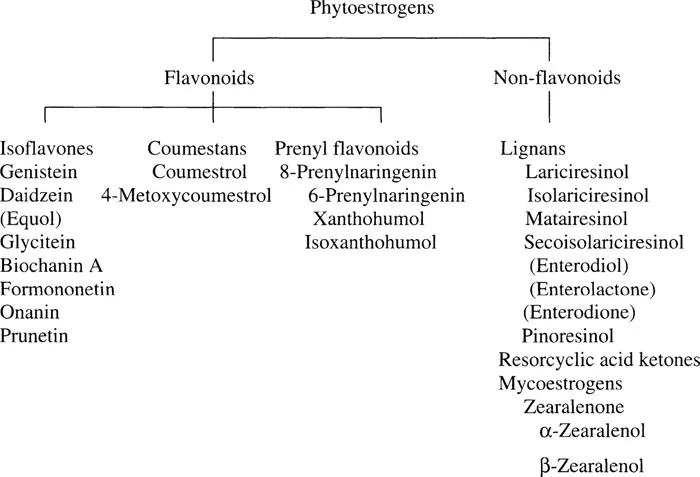
FIGURE 1.1
The relationship between various groups of phytoestrogens (given in bold) and members of each group. (The compounds in parentheses are not inherently present in plants but are oestro-genic products resulting from metabolism of members of that class of phytoestrogens.)
The relationship between various groups of phytoestrogens (given in bold) and members of each group. (The compounds in parentheses are not inherently present in plants but are oestro-genic products resulting from metabolism of members of that class of phytoestrogens.)
TABLE 1.1
Key Structural Elements Crucial for Estradiol-Like Action
Key Structural Elements Crucial for Estradiol-Like Action
Presence of the phenolic ring indispensable for binding to estrogen receptors (ERs) Role of the ring of isoflavones mimicking the ring of estrogens at receptor binding Low molecular weights, similar to that of estradiol (C18H24O2) (MW = 272) Distance between two aromatic hydroxyl groups in the nucleus of the isoflavones almost identical to the distance between two hydroxyl groups of estradiol Optimal pattern of hydroxylation, i.e., hydroxyl substituents at 4, 5, and 7 positions (e.g., genistein) |
The structural similarities between members of the four main groups of phytoestrogens identified in Figure 1.1 and estradiol are shown in Figure 1.2.7
1.1.3 Estrogenic Properties of Phytoestrogens
In the 1940s, it was first realized that some plant-derived compounds could cause estrogenic effects in animals.8 Sheep grazing on pastures containing red clover had multiple fertility problems. The clover in these pastures had high amounts of the isoflavones formononetin and biochanin A.9 The phytoestrogens daidzein and genistein were responsible for the infertility of some captive cheetahs fed a soybean-enriched diet subsequently found to contain high quantities of these compounds.10
Evidence is beginning to accrue that phytoestrogens may begin to offer protection against a wide range of human conditions, including breast, bowel, prostate, and other cancers; cardiovascular disease; brain function; alcohol abuse; osteoporosis; and menopausal symptoms.11 The basis for these effects has not been established, but the weak estrogenic activity of isoflavones may be a factor in conferring these properties.5
The incidence of a number of cancers, including those of the breast and prostate, has been found to be much higher in Western populations compared with that in countries such as Japan and China. Epidemiological and migrant studies have suggested that racial characteristics and other factors including lifestyle, diet, and fat or fiber intake may play a role in the etiology of these diseases. One notable dietary difference is the relatively high consumption of soy and soy-based foods among Asian populations. Comparison of estimated dietary isoflavone intakes in Western and Eastern (e.g., Japanese and Chinese) populations illustrate that Eastern populations have a significantly higher intake of phytoestrogens. Estimates suggest that the average Japanese consumer is exposed to approximately 25 to 100 mg isoflavones/day, while an average United Kingdom consumer ingests approximately 1 mg isoflavones/day.7 As such, soy, which has been known to be the richest source of isoflavones, has attracted much attention as a potential chemopro-tective factor.11,12
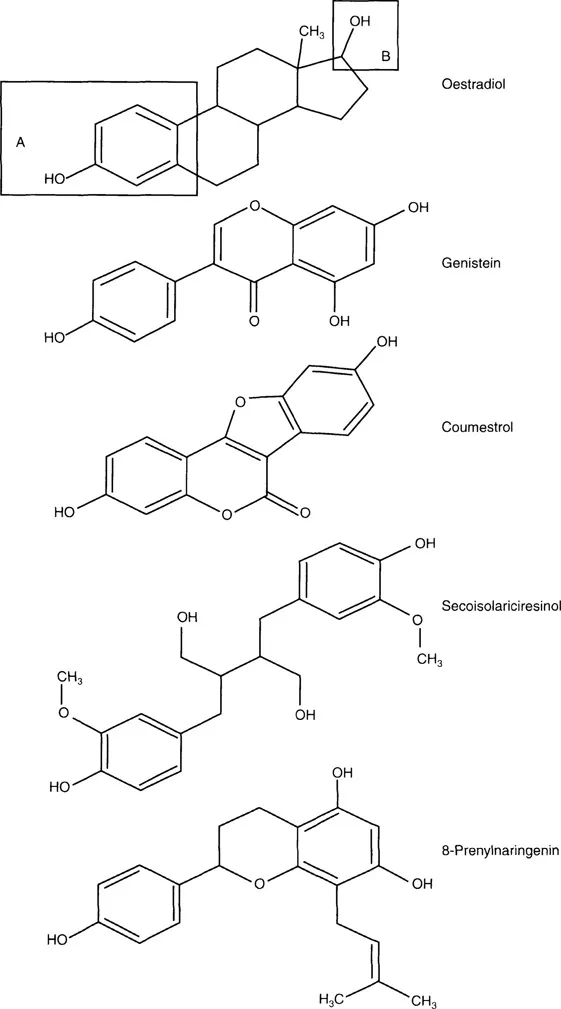
FIGURE 1.2
The structural similarities of phytoestrogens to estradiol. (The similarity of the structure of estradiol and examples from the four classes of phytoestrogens from Figure 1.1. All the structures possess the phenolic [A] and hydroxyl [B] moieties outlined in boxes on the estradiol structure, and the distances between the two groups in each compound are similar.)
The structural similarities of phytoestrogens to estradiol. (The similarity of the structure of estradiol and examples from the four classes of phytoestrogens from Figure 1.1. All the structures possess the phenolic [A] and hydroxyl [B] moieties outlined in boxes on the estradiol structure, and the distances between the two groups in each compound are similar.)
1.1.4 Relative Estrogenic Activity of Phytoestrogens
In general, phytoestrogens are relatively weak oestrogens, requiring much higher concentrations than estradiol to produce an equivalent biological response. Since potency values can vary significantly between methods, relative absolute estrogenic potency of phytoestrogens is difficult to determine. However, taking the results of both in vitro and in vivo studies together, a single rank order of oestrogenic potency of phytoestrogens may be estimated: estradiol coumestrol > genistein, equol > glycitein > 8-prenylnarin-genin > daidzein > formononetin, biochanin A, 6-prenylnaringenin, xanthohumol, isoxanthohumol.7
Because coumestans, reported to be the most potent phytoestrogen,13 are found predominantly in clover and alfalfa plants14 and so are rare components of the human diet,15 isoflavones attract a great deal of interest in today’s studies due to the wider range of foods containing them.
1.2 Isoflavones
Isoflavones are polyphenolic phytoestrogens that occur mainly as gluco-conjugates (glucosides) of genistein, daidzein, and glycitein.16 They enjoy a restricted distribution in the plant kingdom and are predominantly found in leguminous plants.17 The main dietary sources of isoflavones are soybeans and soy foods.18
1.2.1 Classes of Isoflavones
The most prevalent isoflavones present in plant-based foods are as follows11 (Figure 1.3):
- Genistein
- Daidzein
- Glycitein
- Biochanin A (methylated derivative of genistein)
- Formononetin (methylated derivative of daidzein)
Table of contents
- Cover
- Half Title
- Title Page
- Copyright Page
- Table of Contents
- Preface
- Contributor
- Acknowledgments
- Part I Production
- Part II Consumption
- Part III Risk and Benefit Analysis
- Part IV Optimization and Utilization
- Part V Applications
- Glossary of Terms and Abbreviations in Phytoestrogen Research
- Index