
- 1,004 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Phospholipids Handbook
About this book
Employing a multidisciplinary approach to phospholipid research, this work catalogues the current knowledge of this class of molecules and details the general, chemical, physical and structural properties of phospholipid monolayers and bilayers. Phospholipid applications are also covered.
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
I
General and Chemical Properties
1
Structure and Nomenclature
I. INTRODUCTION
An enormous variety of phospholipid structures is found in nature, exhibiting great diversity in the structures of both the apolar and the polar moieties of the lipid molecules. While any individual lipid species may be named according to rigorous rules of organic-chemical nomenclature [1, 5], a practical system of nomenclature for biologically occurring lipids must also offer reasonable brevity and simplicity to be generally useful. Moreover, even a ‘purified’ lipid preparation that is obtained from a biological source may be homogeneous with respect to one structural feature (e.g., the polar portions of the lipid molecules) but highly heterogeneous with respect to another (e.g., their hydrocarbon chains). A useful system of nomenclature for lipids from natural sources must allow for this fact by permitting certain structural features of the lipids in a given preparation to be described in a generic manner while other features of the structure are specified entirely.
Three general types of nomenclature for lipids will be described in this chapter. In what will be referred to as the ‘fully systematic’ or ‘formal’ system of nomenclature, all acyl and alk(en)yl residues are fully specified, using their systematic (IUPAC) designations [1], and all other structural units in the lipid molecule (polyols, monosaccharide units, amino acids, phospho- moieties, etc.) are specified individually and in full, using the nomenclature recommended in the IUPAC-IUB proposals for the Nomenclature of Lipids [4], Nomenclature of Phosphorus-Containing Compounds of Biological Importance [3] and Prenol Nomenclature [5]. In systems of nomenclature referred to below as ‘trivial,’ details of stereochemistry are often absent or implicit, and major portions of the molecule may be named as simple units (e.g., as a ‘phosphatidyl’ group) or generically. Finally, shorthand systems of nomenclature have been developed, using simple alphabetical or numerical symbols to allow concise specification of the structures of even complex lipid molecules (e.g., glycolipids) or to focus on particular details of molecular structure, such as the fatty acyl composition of a natural lipid preparation.
It is common in practice to define completely the structures of both the apolar and the polar portions of pure synthetic phospholipids, which constitute single molecular species. By contrast, it is common to specify in detail only the polar portions of ‘pure’ lipid fractions from natural sources while designating the apolar portions, which are normally heterogeneous in composition, in a generic manner. Accordingly, this chapter will first describe the systems of nomenclature used to define the structures of the apolar and ‘backbone’ portions of lipid molecules. The following sections describe systems of nomenclature that are appropriate to describe either individual phospholipid molecular species or phospholipid fractions that are uniform in some structural features but heterogeneous in others.
II. APOLAR AND ‘BACKBONE’ MOIETIES OF PHOSPHOLIPIDS
Almost all biologically occurring phospholipids are constructed from two combinations of apolar and ‘backbone’ moieties: a glycerol (or other polyol) moiety substituted with one or two acyl or alkyl chains; or an N-acylated sphingoid base (i.e., a ceramide).
A. Structure and Nomenclature of Acyl/Alkylated Glycerol Moieties
While glycerol itself is a symmetrical molecule, its carbon-2 becomes a chiral center when the 1- and 3-positions are not symmetrically substituted. It is therefore useful to define a prochiral ‘sn-glycerol’ (sn = stereospecific numbering [6]) in which the orientation of the 2-hydroxyl group and the numbering of the carbons are as shown in Fig. 1. In virtually all natural phospholipids, excepting certain archaebacterial lipids discussed below, the polar headgroup is attached to the 3-position of ‘sn-glycerol,’ in which the configuration of substituents about C-2 would be designated as R in the (R,S)-system. The ‘sn-’ convention is also applied to describe the configuration of other glycerol residues in phospholipid molecules (e.g., for the biologically occurring 1-(1',2'-diacyl-sn-glycero-3-phospho)-sn-glycerol and its 3-O-amino acyl esters [7]). Glycerol residues that are not either symmetrically or stereospecifically substituted are designated using the prefix rac-, as in 1-(1', 2'-diacyl-sn-glycero-3'-phospho)-rac-glycerol, in which the nonacylated glycerol comprises a mixture of 1- and 3-substituted ‘sn-glycerol’ residues.
1. Acyl Residues
Acyl chains are named formally using standard IUPAC rules of nomenclature for organic compounds [1], although certain features of the IUPAC system, notably the use of the (E,Z)-convention to designate the configuration about the double bond, have not received widespread use in lipid nomenclature. The systematic name of an acyl residue is derived from that of the corresponding fatty acid by replacing the suffix ‘-oic acid-’ by ‘-oyl,’ as in hexadecanoyl or cis, cis-9, 12-octadecadienoyl.
Most of the fatty acids found in natural phospholipids also have one or more trivial names (Table 1; for more extensive compilations see [8,9]). To designate the name of an acyl residue using the trivial nomenclature, the suffix ‘-ic acid’ in the common name of the parent fatty acid is replaced by the suffix ‘-oyl,’ in analogy to the designation of acyl residues in the IUPAC system. This rule can lead to confusion in certain cases, however, notably for decanoyl chains, which it designated as ‘caproyl’ (from capric acid), could be mistaken for a residue of esterified hexanoic (caproic) acid.
Several common systems also exist for the shorthand designation of fatty acyl chains. The first, which is used in an IUPAC-IUB-recommended system for the shorthand designation of lipid structures [4], consists of a set of abbreviations (Pam = palmitoyl or hexadecanoyl, Lin = linoleoyl = or cis, cis-9, 12-octadecadienoyl, etc.) for common alkanoyl and alkenoyl chains. A listing of the shorthand designations of common fatty acids in this system is given in Table 1.
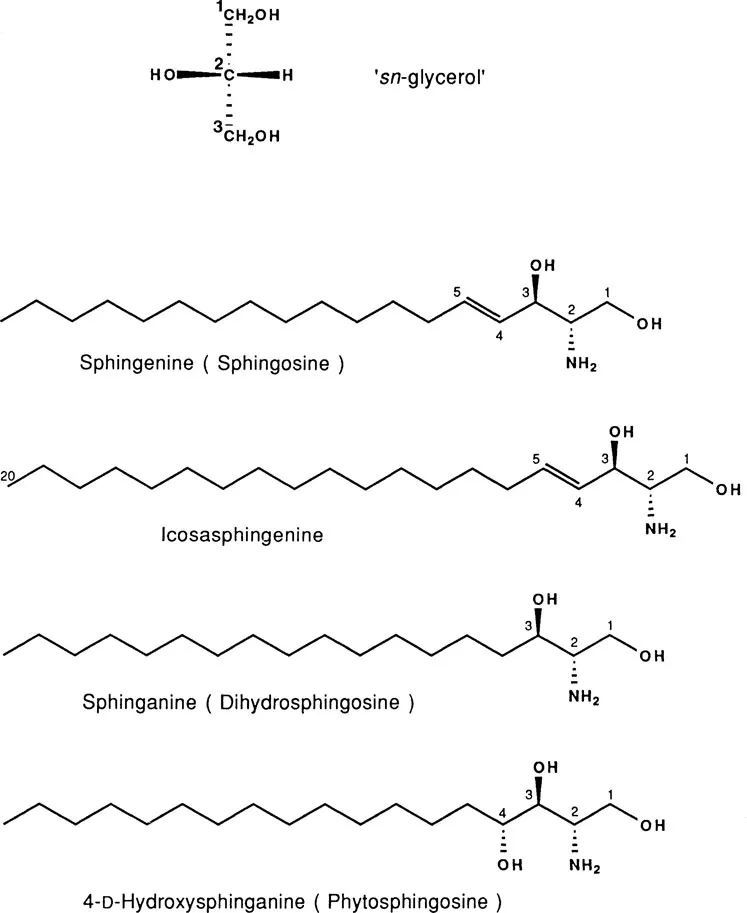
Figure 1 Structures of sn-glycerol and of the major sphingoid bases.
Table 1 Systematic, Trivial, and Shorthand Designations for Some Common Fatty Acyl Chains of Phospholipids
Shorthand designations | ||||
Systematic name | Common name | IUPAC-IUB | Δ-system | n-system |
Dodecanoyl | Lauroyl | Lau | 12:0 | 12:0 |
Tetradecanoyl | Myristoyl | Myr | 14:0 | 14:0 |
Hexadecanoyl | Palmitoyl | Pam | 16:0 | 16:0 |
Octadecanoyl | Stearoyl | Ste | 18:0 | 18:0 |
Icosanoyl | Arachidoyl | Ach | 20:0 | 20:0 |
cis-9-Octadecenoyl | Oleoyl | Ole | 18:1cΔ9 | 18:1(n-9) |
cis,cis-9, 12-Octadeca dienoyl | Linoleoyl | Lin | 18:2ccΔ9, 12 | 18:2(n-6) |
all-cis-9, 12, 15-Octadecatrienoyl | γ-Linolenoyl | Lnn | 18:3cccΔ9, 12, 15 | 18:3(n-3) |
all-cis-5, 8, 11, 14-Icosatetraenoyl | Arachidonoyl | Δ4Ach | 20:4ccccΔ5, 8, 11, 14 | 20:4(n-6) |
Other more systematic systems for the shorthand representation of fatty acyl residues are frequently used in tabulations of the fatty acyl compositions of lipid preparations. In most systems of this type, the carbon number of the fatty acid is indicated first, followed after a colon b...
Table of contents
- Cover
- Half Title
- Title Page
- Copyright Page
- Table of Contents
- Preface
- Contributors
- Part I: General and Chemical Properties
- Part II: Physical and Structural Properties
- Part III: Biological Aspects
- Appendix A: Structural Parameters of Phospholipids
- Appendix B: Thermodynamic Parameters of Phospholipids
- Appendix C: Mechanical, Solubility, and Related Parameters of Phospholipids
- Index
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Phospholipids Handbook by Gregor Cevc in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Biochemistry in Medicine. We have over one million books available in our catalogue for you to explore.