
eBook - ePub
Phospholipids Handbook
Gregor Cevc, Gregor Cevc
This is a test
Partager le livre
- 1,004 pages
- English
- ePUB (adapté aux mobiles)
- Disponible sur iOS et Android
eBook - ePub
Phospholipids Handbook
Gregor Cevc, Gregor Cevc
Détails du livre
Aperçu du livre
Table des matières
Citations
À propos de ce livre
Employing a multidisciplinary approach to phospholipid research, this work catalogues the current knowledge of this class of molecules and details the general, chemical, physical and structural properties of phospholipid monolayers and bilayers. Phospholipid applications are also covered.
Foire aux questions
Comment puis-je résilier mon abonnement ?
Il vous suffit de vous rendre dans la section compte dans paramètres et de cliquer sur « Résilier l’abonnement ». C’est aussi simple que cela ! Une fois que vous aurez résilié votre abonnement, il restera actif pour le reste de la période pour laquelle vous avez payé. Découvrez-en plus ici.
Puis-je / comment puis-je télécharger des livres ?
Pour le moment, tous nos livres en format ePub adaptés aux mobiles peuvent être téléchargés via l’application. La plupart de nos PDF sont également disponibles en téléchargement et les autres seront téléchargeables très prochainement. Découvrez-en plus ici.
Quelle est la différence entre les formules tarifaires ?
Les deux abonnements vous donnent un accès complet à la bibliothèque et à toutes les fonctionnalités de Perlego. Les seules différences sont les tarifs ainsi que la période d’abonnement : avec l’abonnement annuel, vous économiserez environ 30 % par rapport à 12 mois d’abonnement mensuel.
Qu’est-ce que Perlego ?
Nous sommes un service d’abonnement à des ouvrages universitaires en ligne, où vous pouvez accéder à toute une bibliothèque pour un prix inférieur à celui d’un seul livre par mois. Avec plus d’un million de livres sur plus de 1 000 sujets, nous avons ce qu’il vous faut ! Découvrez-en plus ici.
Prenez-vous en charge la synthèse vocale ?
Recherchez le symbole Écouter sur votre prochain livre pour voir si vous pouvez l’écouter. L’outil Écouter lit le texte à haute voix pour vous, en surlignant le passage qui est en cours de lecture. Vous pouvez le mettre sur pause, l’accélérer ou le ralentir. Découvrez-en plus ici.
Est-ce que Phospholipids Handbook est un PDF/ePUB en ligne ?
Oui, vous pouvez accéder à Phospholipids Handbook par Gregor Cevc, Gregor Cevc en format PDF et/ou ePUB ainsi qu’à d’autres livres populaires dans Sciences physiques et Chimie organique. Nous disposons de plus d’un million d’ouvrages à découvrir dans notre catalogue.
Informations
I
General and Chemical Properties
1
Structure and Nomenclature
I. INTRODUCTION
An enormous variety of phospholipid structures is found in nature, exhibiting great diversity in the structures of both the apolar and the polar moieties of the lipid molecules. While any individual lipid species may be named according to rigorous rules of organic-chemical nomenclature [1, 5], a practical system of nomenclature for biologically occurring lipids must also offer reasonable brevity and simplicity to be generally useful. Moreover, even a ‘purified’ lipid preparation that is obtained from a biological source may be homogeneous with respect to one structural feature (e.g., the polar portions of the lipid molecules) but highly heterogeneous with respect to another (e.g., their hydrocarbon chains). A useful system of nomenclature for lipids from natural sources must allow for this fact by permitting certain structural features of the lipids in a given preparation to be described in a generic manner while other features of the structure are specified entirely.
Three general types of nomenclature for lipids will be described in this chapter. In what will be referred to as the ‘fully systematic’ or ‘formal’ system of nomenclature, all acyl and alk(en)yl residues are fully specified, using their systematic (IUPAC) designations [1], and all other structural units in the lipid molecule (polyols, monosaccharide units, amino acids, phospho- moieties, etc.) are specified individually and in full, using the nomenclature recommended in the IUPAC-IUB proposals for the Nomenclature of Lipids [4], Nomenclature of Phosphorus-Containing Compounds of Biological Importance [3] and Prenol Nomenclature [5]. In systems of nomenclature referred to below as ‘trivial,’ details of stereochemistry are often absent or implicit, and major portions of the molecule may be named as simple units (e.g., as a ‘phosphatidyl’ group) or generically. Finally, shorthand systems of nomenclature have been developed, using simple alphabetical or numerical symbols to allow concise specification of the structures of even complex lipid molecules (e.g., glycolipids) or to focus on particular details of molecular structure, such as the fatty acyl composition of a natural lipid preparation.
It is common in practice to define completely the structures of both the apolar and the polar portions of pure synthetic phospholipids, which constitute single molecular species. By contrast, it is common to specify in detail only the polar portions of ‘pure’ lipid fractions from natural sources while designating the apolar portions, which are normally heterogeneous in composition, in a generic manner. Accordingly, this chapter will first describe the systems of nomenclature used to define the structures of the apolar and ‘backbone’ portions of lipid molecules. The following sections describe systems of nomenclature that are appropriate to describe either individual phospholipid molecular species or phospholipid fractions that are uniform in some structural features but heterogeneous in others.
II. APOLAR AND ‘BACKBONE’ MOIETIES OF PHOSPHOLIPIDS
Almost all biologically occurring phospholipids are constructed from two combinations of apolar and ‘backbone’ moieties: a glycerol (or other polyol) moiety substituted with one or two acyl or alkyl chains; or an N-acylated sphingoid base (i.e., a ceramide).
A. Structure and Nomenclature of Acyl/Alkylated Glycerol Moieties
While glycerol itself is a symmetrical molecule, its carbon-2 becomes a chiral center when the 1- and 3-positions are not symmetrically substituted. It is therefore useful to define a prochiral ‘sn-glycerol’ (sn = stereospecific numbering [6]) in which the orientation of the 2-hydroxyl group and the numbering of the carbons are as shown in Fig. 1. In virtually all natural phospholipids, excepting certain archaebacterial lipids discussed below, the polar headgroup is attached to the 3-position of ‘sn-glycerol,’ in which the configuration of substituents about C-2 would be designated as R in the (R,S)-system. The ‘sn-’ convention is also applied to describe the configuration of other glycerol residues in phospholipid molecules (e.g., for the biologically occurring 1-(1',2'-diacyl-sn-glycero-3-phospho)-sn-glycerol and its 3-O-amino acyl esters [7]). Glycerol residues that are not either symmetrically or stereospecifically substituted are designated using the prefix rac-, as in 1-(1', 2'-diacyl-sn-glycero-3'-phospho)-rac-glycerol, in which the nonacylated glycerol comprises a mixture of 1- and 3-substituted ‘sn-glycerol’ residues.
1. Acyl Residues
Acyl chains are named formally using standard IUPAC rules of nomenclature for organic compounds [1], although certain features of the IUPAC system, notably the use of the (E,Z)-convention to designate the configuration about the double bond, have not received widespread use in lipid nomenclature. The systematic name of an acyl residue is derived from that of the corresponding fatty acid by replacing the suffix ‘-oic acid-’ by ‘-oyl,’ as in hexadecanoyl or cis, cis-9, 12-octadecadienoyl.
Most of the fatty acids found in natural phospholipids also have one or more trivial names (Table 1; for more extensive compilations see [8,9]). To designate the name of an acyl residue using the trivial nomenclature, the suffix ‘-ic acid’ in the common name of the parent fatty acid is replaced by the suffix ‘-oyl,’ in analogy to the designation of acyl residues in the IUPAC system. This rule can lead to confusion in certain cases, however, notably for decanoyl chains, which it designated as ‘caproyl’ (from capric acid), could be mistaken for a residue of esterified hexanoic (caproic) acid.
Several common systems also exist for the shorthand designation of fatty acyl chains. The first, which is used in an IUPAC-IUB-recommended system for the shorthand designation of lipid structures [4], consists of a set of abbreviations (Pam = palmitoyl or hexadecanoyl, Lin = linoleoyl = or cis, cis-9, 12-octadecadienoyl, etc.) for common alkanoyl and alkenoyl chains. A listing of the shorthand designations of common fatty acids in this system is given in Table 1.
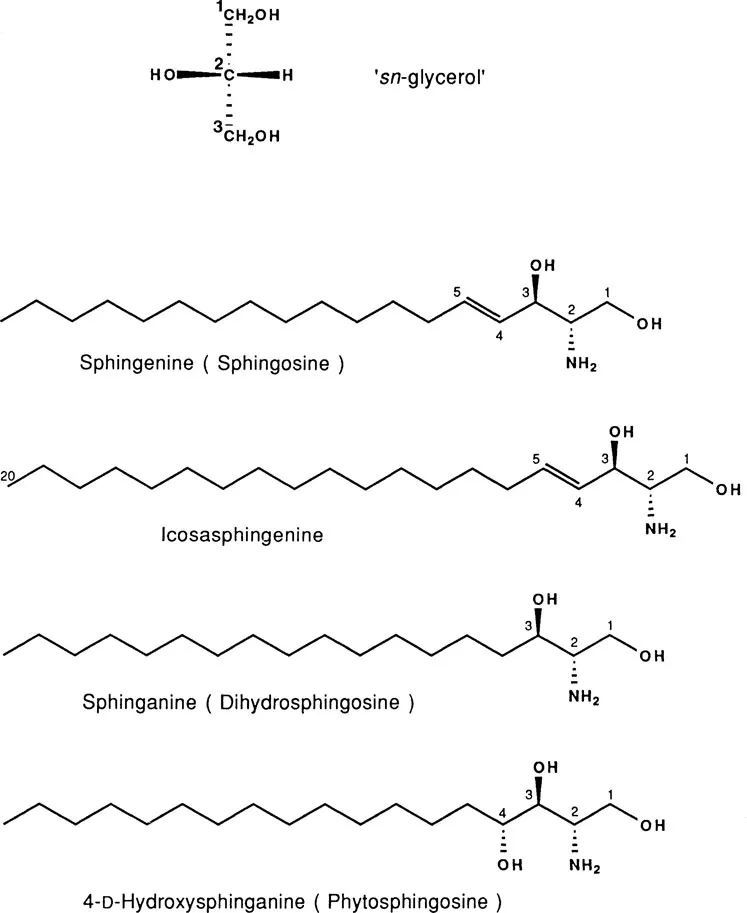
Figure 1 Structures of sn-glycerol and of the major sphingoid bases.
Table 1 Systematic, Trivial, and Shorthand Designations for Some Common Fatty Acyl Chains of Phospholipids
Shorthand designations | ||||
Systematic name | Common name | IUPAC-IUB | Δ-system | n-system |
Dodecanoyl | Lauroyl | Lau | 12:0 | 12:0 |
Tetradecanoyl | Myristoyl | Myr | 14:0 | 14:0 |
Hexadecanoyl | Palmitoyl | Pam | 16:0 | 16:0 |
Octadecanoyl | Stearoyl | Ste | 18:0 | 18:0 |
Icosanoyl | Arachidoyl | Ach | 20:0 | 20:0 |
cis-9-Octadecenoyl | Oleoyl | Ole | 18:1cΔ9 | 18:1(n-9) |
cis,cis-9, 12-Octadeca dienoyl | Linoleoyl | Lin | 18:2ccΔ9, 12 | 18:2(n-6) |
all-cis-9, 12, 15-Octadecatrienoyl | γ-Linolenoyl | Lnn | 18:3cccΔ9, 12, 15 | 18:3(n-3) |
all-cis-5, 8, 11, 14-Icosatetraenoyl | Arachidonoyl | Δ4Ach | 20:4ccccΔ5, 8, 11, 14 | 20:4(n-6) |
Other more systematic systems for the shorthand representation of fatty acyl residues are frequently used in tabulations of the fatty acyl compositions of lipid preparations. In most systems of this type, the carbon number of the fatty acid is indicated first, followed after a colon b...