![]()
1 Nutritional and Environmental Influences on Brain Development
Critical Periods of Brain Development, Pathways, and Mechanisms of Effect
Bryan Kolb, Celeste Halliwell, and Robbin Gibb
CONTENTS
1.1 Introduction
1.2 Brain Development in Humans and Rodents
1.3 Special Features of Brain Development
1.3.1 Stem Cells
1.3.2 Plasticity
1.4 Factors Influencing Brain Development in the Normal Brain
1.4.1 Sensory and Motor Experiences
1.4.2 Psychoactive Drugs
1.4.3 Stress
1.4.4 Peer Relationships
1.4.5 Parent–Child Relationships
1.4.6 Intestinal Flora
1.4.7 Early Brain Injury
1.4.8 Nutrition and Brain Development
1.5 Nutrition and Early Brain Injury
1.6 Conclusion
Acknowledgement
References
1.1 INTRODUCTION
Brain and behavioural development is influenced by a wide range of factors, including nutrients. Although there is a considerable literature on the effects of early life nutrition on normal and abnormal behavioural development (e.g. Georgieff 2007; Georgieff & Rao 2001; Leung et al. 2011; Rucklidge & Kaplan 2013), far less is known about how early nutrition affects either brain plasticity or the effects of perinatal brain injury. The goal of this chapter is to introduce the reader to these issues. We begin with a brief review of brain development and plasticity, consider a model of early brain injury in rats, and then consider the role of nutrition in stimulating recovery and brain plasticity.
1.2 BRAIN DEVELOPMENT IN HUMANS AND RODENTS
Brain development is a long and complex process that can broadly be divided into two phases in mammals. The first phase is in utero and reflects a genetically determined sequence of events that are modulated by the internal maternal environment and external factors that influence this environment. The second phase of the development is largely postnatal in species such as the rat but occurs in both pre- and postnatal periods in species such as humans. This phase represents a period where the developing connectivity and organization of the brain is very sensitive to internal and external environmental stimuli.
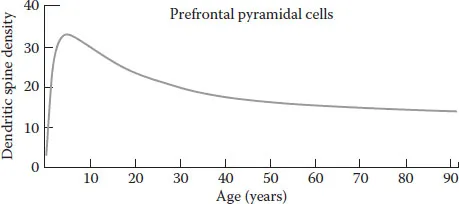
Table 1.1 summarizes seven general stages of brain development characteristic of all mammals (for extensive reviews, see Molnar & Clowry 2012; Semple et al. 2013). The generation of neurons begins around 6 weeks after conception in humans and on embryonic (E) day 10.5–11 in rats. Neurogenesis in the cerebral cortex is largely complete in humans around 30 weeks and in rats by birth (E21–22). Neuronal migration begins after neuron generation, and in humans, it continues for about 6 weeks and in rats until about postnatal (P) day 5. Cell differentiation is essentially complete at birth in humans, although neuron maturation, which includes the growth of dendrites, axons, and synapses, goes on for years. Synapse formation in the human cerebral cortex is a daunting challenge for the brain, with a total of more than 100,000 trillion (1014). This enormous number could not possibly reflect a predetermined genetic programme but rather reflects an unusual process of overproduction of both neurons and synapses that are pruned back by a variety of environmental cues and signals (see Figure 1.1).
TABLE 1.1
Stages of Brain Development
1. Cell birth (neurogenesis, gliogenesis) |
2. Cell migration |
3. Cell differentiation |
4. Cell maturation (dendrite and axon growth) |
5. Synaptogenesis (formation of synapses) |
6. Cell death and synaptic pruning |
7. Myelogenesis (formation of myelin) |
FIGURE 1.1 Spine pruning in the dorsolateral prefrontal cortex of humans. Spine density in layer III pyramidal cells reaches a peak around age 5, and then declines into the 30s, before stabilizing at adult levels. (Data from Petanjek, Z. et al., Proc. Natl. Acad. Sci. USA, 108, 13283, 2011, Figure 1.2b.)
The pruning of synapses follows, beginning in the visual cortex after 1 year but not until a couple of years later in some parts of the brain such as the prefrontal cortex (e.g. Huttenlocher 1984; Petanjek et al. 2011). Cell differentiation peaks around P7–10 in rats, followed by a rapid increase in synapse production from about P10 to P30, depending upon the brain region. Synaptic pruning is not well studied in the rat, but Van Eden et al. (1990) showed a decline in cortical thickness in the prefrontal cortex from P60 to P90, and Vinish et al. (2013) showed a decrease in spine density over a similar time period in the medial prefrontal cortex (mPFC).
One surprising effect of pruning, as well as other factors, is that the cortex actually becomes measurably thinner in a caudal–rostral gradient in humans, beginning around age 2 and continuing until at least 20 years of age (e.g. O’Hare & Sowell 2008). Using structural MRI scans, Houston et al. (2014) examined the relationship between reading skill and cortical grey matter volume change in boys aged 5–15 years who received two scans about 2 years apart. The authors found an inverse correlation between improved scores on various reading-related cognitive tests and reduced volume of grey matter in the left inferior parietal cortex and left inferior frontal cortex. It appeared that children who were better readers had a different trajectory of cortical maturation than those who read less well.
One important additional aspect for understanding brain development is the effect of epigenetics, which is the heritable change in gene expression that does not result from changes in the sequence of DNA. Epigenetic mechanisms create variation in phenotypes, without altering the base-pair nucleotide sequence of the genes. Thus, the environment can allow a gene to be expressed or to be prevented from its expression. Although epigenetic mechanisms are at work throughout the lifetime, they are especially important during brain development: our early experiences induce changes in our brains that make us unique. For example, developing brains exposed to different environmental events such as sensory stimuli, stress, injury, diet, drugs, gut bacteria, peer play, and social relationships can show dramatically different phenotypes later in life. Furthermore, there is growing evidence that epigenetic changes can cross generations. One especially powerful influence is gestational stress, which affects not only epigenetics and behaviour in the offspring but also their offspring (see the review by Babenko et al. 2015).
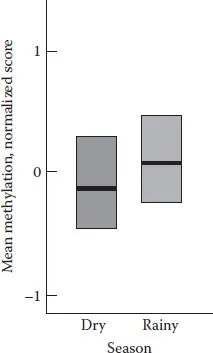
FIGURE 1.2 Diet and gene expression. The diets of women in rural Gambia vary with the season. Babies conceived in the rainy season show significantly higher gene methylation (fewer genes expressed) compared with babies conceived in the dry season. The dark horizontal bar represents the median and the box is the range. (Data from Dominguez-Salas et al. 2014, Figure 1.3b.)
A maternal diet during gestation has long been known to alter the offspring’s brain development and later behaviour, but the mechanisms are only beginning to be examined. For example, Dominguez-Salas et al. (2014) showed that maternal diet at conception significantly altered gene methylation in newborns (i.e. gene methylation is a measure of epigenetic change). The researchers studied infants in rural Gambia who had been conceived in either the dry season or the rainy season. Gambians’ diets are dramatically different during these two seasons and so was gene methylation in the infants’ blood (see Figure 1.2). The level of gene methylation reflects the number of genes expressed. Increased gene methylation, for instance, means that fewer genes are expressed, and thus, the body and brain will develop differently.
1.3 SPECIAL FEATURES OF BRAIN DEVELOPMENT
Two features of brain development are especially important in understanding how experiences can modify cerebral organization: stem cells and plasticity.
1.3.1 STEM CELLS
The cells residing in the subgranular zone (SGZ) in the hippocampus are stem cells that remain active throughout life, producing neurons and astrocytes at a fairly stable rate, although there is a decline with senescence. The cells lining the subventricular zone (SVZ) lying below the neocortex can produce neural or glial progenitor cells that are capable of migrating into the white or grey matter throughout life. These cells remain quiescent for extended periods of time, however, and their role remains poorly understood, but their generation is influenced by many factors including experience, drugs, hormones, and injury.
1.3.2 PLASTICITY
Brain plasticity refers to the capacity of the brain to change its structure and ultimately its function (e.g. Kolb 1995). Changes in the brain can be shown at many levels of analysis ranging from molecules to behaviour. One advantage of using laboratory animals is that it is possible to measure anatomical and molecular changes in post-mortem tissue of animals with different experiences and to correlate these changes with behaviour.
Three types of plasticity can be distinguished in the normal developing brain: experience-independent, experience-expectant, and experience-dependent plasticity (Greenough et al. 1987; Shatz 1992). Experience-independent plasticity is largely a prenatal developmental process. It is impractical for the genome to specify the connectivity of every connection in neuron development. Instead, the brain produces a rough structure in which there is an overproduction of neurons and, later, connections, which are sculpted in response to internal and external events. A good example is the development of the eye-specific layers of the lateral geniculate nucleus (LGN) of the cat (Campbell & Shatz 1992). The mature LGN has several layers that receive specific connections from each eye. To segregate the layers correctly, the retinal ganglion cells spontaneously fire so as to correlate their firing with nearby cells in the same eye but independent of those in the other eye. Cells which are active together increase their connections, whereas those out of synch weaken their connections and eventually die out. Experience-independent plasticity allows the nervous system more precision in connectivity without requiring overwhelmingly complex genetic instructions.
Experience-expectant plasticity largely occurs during development, often during sensitive periods. For example, the human brain expects to hear speech sounds, but it typically hears only those of one or two languages. Because different languages are composed of language-specific sounds, the early exposure to these sounds activates certain groups of neurons, which are sustained by the activity, whereas other sounds are not encountered, and the ability to distinguish them is reduced. Later in life, it becomes difficult to learn to distinguish certain sounds in other languages, even though all babies can do so, leading sometimes to strong accents in adults learning new languages.
Finally, experience-dependent plasticity refers to a process of changing neuronal ensembles that are growing or already present. This can be seen in situations such as when animals learn problems (e.g. Comeau et al. 2010; Greenough & Chang 1989), when animals receive intense environmental manipulations (e.g. Greenough & Chang 1989), have brain injury (e.g. Kolb et al. 2013), or response to psychoactive drugs (e.g. Robinson & Kolb 2004). These types of experiences can both increase and decrease synapse numbers concurrently but in different regions in the same animals. Although experience-dependent plasticity occurs throughout the lifespan, it occurs extensively during development and will influence both how a brain responds to injury and post-injury interventions.
Two features of brain plasticity are especially important in the current context. First, plastic changes are age-dependent. It is generally expected that the developing brain will be more responsive to experiences than the adult or senescent brain, and this is true. One unexpected age-related effect, however, is that there are qualitatively different plastic changes in the brain at different ages. One of the most powerful ways to change the brain of the laboratory rat is to place it in a complex environment which provides extensive social interaction and activity. Rats placed in such environments as adults show a widespread increase in spine density, reflecting an increase in excitatory synapses. In contrast, rats placed in the same environments as juveniles show a decrease in spine density, although the cells remain the same size (Kolb et al. 2003). Importantly, both groups of stimulated animals show similar enhanced cognitive and motor capacities. A parallel example comes from the effects of nicotine. When administered in adulthood, nicotine increased spine density in the medial frontal cortex, but when administered prenatally, it decreased spine density (Brown & Kolb 2001; Mychasiuk et al. 2013a). A final example can be seen in the effects of early cortical injury. In rats, injury in the first few days of life drastically reduced neuronal complexity and spine density in the cortex, whereas a similar injury in the second week of life produced the opposite effect (e.g. Kolb & Gibb 20...