![]()
1
Calorimetry of Pre- and Postextruded Cereal Flours
Gönül Kaletunç
The Ohio State University, Columbus, Ohio, U.S.A.
Kenneth J. Breslauer
Rutgers University, Piscataway, New Jersey, U.S.A.
I. INTRODUCTION
Extrusion processing is widely utilized in the food and feed industries for the manufacture of value-added products. Extrusion processing is a versatile technology producing a wide range of products, including confectionery products, pasta, ready-to-eat (RTE) cereals, flat bread, snack products, texturized proteins, and pet foods. A broad range of operating parameters is used to manufacture products with a large variety of structures and textures, ranging from high moisture (up to 75%)–low temperature (as low as 50°C)–low shear in texturized vegetable and pasta production to low moisture (as low as 11%)–high temperature (as high as 180°C)–high shear in breakfast cereal and snack production.
High-temperature extrusion processing finds wide application in the food industry for the preparation of breakfast cereals and snack foods. Starch-and protein-based cereal flours are frequently encountered as major components of the raw material mixtures. Rice, wheat, oat, corn, and mixed grain cereal flours or meals are commonly utilized for extrusion processing. During extrusion, as a result of shear and high temperatures, usually above 140°C, cereal flours are transformed into viscoelastic melts. Upon extrusion, the melt expands and cools rapidly due to vaporization of moisture, eventually settling into an expanded solid foam. Because extrusion processing is associated with thermal manipulation (mainly heating and some cooling for unexpanded materials) of the materials, thermal characterization of cereal flours and their biopolymer components will lead to data that can be related directly to the processing protocols. Furthermore, thermal characterization of extruded products as a function of storage conditions (relative humidity–temperature) allows evaluation of the impact of such treatment.
In this chapter, we review the characterization by calorimetry of thermally induced conformational changes and phase transitions in pre- and postextruded cereal flours and the use of calorimetric data to elucidate the macromolecular modifications that these materials undergo during extrusion processing. The use of calorimetric data as a tool to evaluate the impact of formulation, processing, and storage on end-product attributes will be demonstrated.
II. CALORIMETRY
Differential scanning calorimetry (DSC) is a thermal analysis technique that detects and monitors thermally induced conformational transitions and phase transitions as a function of temperature. A pair of matching crucibles or sample pans, one containing the sample and one serving as reference, are heated in tandem. As a crucible is heated, its temperature increases, depending on the heat capacity of the contents of the crucible. At temperatures where an endothermic transition occurs, the thermal energy supplied to the crucible is consumed by that transition and the temperature of the sample cell lags behind the reference cell temperature. Conversely, the reference cell temperature lags when an exothermic transition occurs in the sample. A temperature difference between the cells results in heat flow between the cells. DSC measures the differential heat flow between the sample and reference crucibles as a function of temperature at a fixed heating rate. DSC thermograms are normalized to yield the specific heat capacity (Cp) as a function of temperature (1).
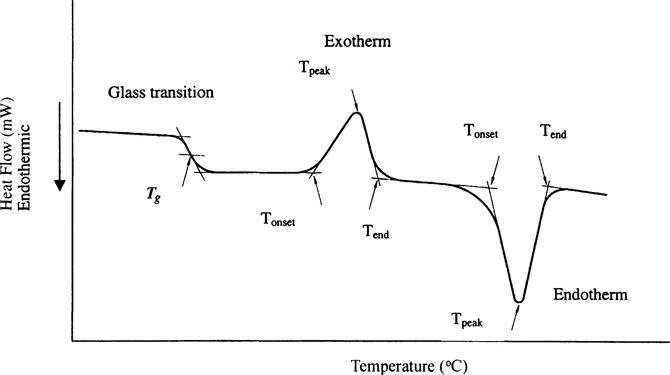
At temperatures where crystalline regions of cereal flour components undergo order–disorder transitions, peaks are observed in the heat flow vs. temperature diagrams, either as heat absorption (endotherm) or as heat release (exotherm). Endotherms are typically associated with the melting of mono-, di-, oligo-, and polysaccharides, denaturation of proteins, and gelatinization of starch. Exotherms are observed for crystallization of carbohydrates and aggregation of denatured proteins. When both crystalline and amorphous structures are present, which is typical in cereal flours, an additional transition is observed prior to the exothermic and endothermic transitions. This transition, known as a glass transition, is associated with amorphous materials or amorphous regions of partially crystalline materials. With DSC, the glass transition is observed as a sharp decrease of the heat capacity on cooling and a sudden increase in heat capacity on heating. A typical DSC thermogram, displaying glass, endothermic, and exothermic transitions, is given in Figure 1.
The glass transition temperature indicates a change in the mobility of the molecular structure of materials. Because cooperative motions in the molecular structure are frozen below the glass transition temperature, for partially crystalline materials exothermic and endothermic events are not observed until the glass transition is completed. Slade and Levine (2) discussed in detail that crystallization (exothermic event) can occur only in the rubbery state and the overall rate of crystallization (net rate of nucleation and propagation) in polymer melts is maximized at a temperature midway between the glass transition and melting temperatures. Furthermore, it has been demonstrated that for partially crystalline polymers the ratio of the melting to glass transition temperatures (Tm/Tg) varies from 0.8 to greater than 1.5. This ratio is shown to correlate with the glass-forming tendency and crystallizability of the polymers, because it predicts the relative mobilities of polymers at Tg and at T ≫ Tg. More specifically, polymers with Tm/Tg ≫ 1.5 readily crystallize, while the polymers with Tm ≪ 1.5 have a high glass-forming tendency. Food biopolymers such as gelatin, native starch, and dimers or monomers such as galactose and fructose are reported to exhibit behavior similar to synthetic polymers with Tm/Tg ≪ 1.5, which demonstrates a large free-volume requirement and thus a large temperature increase required for mobility.
Figure 1 Typical DSC curve for partially crystalline materials.
In DSC thermograms similar to the one in Figure 1, the glass transition is detectable by a step change of the heat capacity. Although Tg can be observed experimentally by measuring physical, mechanical, or electrical properties, it is important to point out that DSC alone supplies thermodynamic information about Tg (3). The thermodynamic property of interest in DSC measurements is the change in the heat capacity, which reflects changes in molecular motions. It should be emphasized that the formation and behavior of the glassy state is a kinetic phenomenon. However, the rubbery state on the high-temperature side of the glass transition is at equilibrium and can be described by equilibrium thermodynamics. Equilibrium thermodynamics also can be applied well below the glass transition temperature because the response of internal degrees of freedom to external effects is very slow. However, during the glass transition, both intrinsic and measurement variables occur on the same time scale, the measured quantities become time dependent, and equilibrium thermodynamics cannot be applied to analyze the system. The system has a memory of its thermal history, which results in the occurrence of relaxation phenomena if the heating and cooling rates are different. It is not possible to get equilibrium values for Tg and the heat capacity change at the glass transition by extrapolating to zero scanning rate because these quantities depend on the thermal history, which includes the scanning rate, annealing temperature, and time.
A complete characterization of the glass transition can be achieved using several parameters. These parameters, as described by Höhne et al. (1), include the temperatures corresponding to vitrification (Tg,f) and devitrification (Tg,i) of the material upon cooling and heating, extrapolated onset temperature (Tg, e), heat capacity change, and the temperature corresponding to the midpoint of the heat capacity change between the extrapolated heat capacity of the glassy and rubbery states (Tg,1/2). The specific heat capacity versus temperature curve derived from a DSC thermogram of a typical glass transition and the parameters describing the glass transition are given in Figure 2.
Tg is also reported as the inflection point of the heat capacity versus temperature curve. The inflection point that corresponds to Tg is the temperature corresponding to the peak in the dCp/dT vs....