
- 220 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
About this book
A review of what needs to be done to realise the potential of monoclonal antibodies. The book assesses the competing technologies with advice on the best approach for a particular situation.
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
At the moment all of our mobile-responsive ePub books are available to download via the app. Most of our PDFs are also available to download and we're working on making the final remaining ones downloadable now. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Monoclonal Antibodies by Heddy Zola in PDF and/or ePUB format, as well as other popular books in Medicine & Immunology. We have over one million books available in our catalogue for you to explore.
Information
Chapter 1
Introduction
1. Introduction: the purpose of this book
The first monoclonal antibodies were described in 1975 (Kohler and Milstein, 1975, 1976), and the first antibodies made with particular applications in mind followed in 1977–78 (Barnstable et al., 1978; Galfre et al., 1977; White et al., 1978; Williams et al., 1977). The potential use of monoclonal antibodies as reagents was soon appreciated widely, leading to rapid growth in the field. Some laboratories turned their entire resources over to the preparation and characterization of monoclonal antibodies, a new sector of the reagents and biotechnology industry developed around monoclonal antibodies, and many immunoassays were greatly improved by the use of monoclonal antibodies. There are now entire fields of research and diagnosis which would be impossible without monoclonal antibodies (the detailed analysis of leukocyte phenotype (Knapp et al., 1989; McMichael et al., 1987; Schlossman et al., 1994) provides just one example). The therapeutic application of monoclonal antibodies, whilst still limited in scope, promises to break its substantial shackles and realize the potential forecast by its proponents.
Most of this has been achieved using techniques which have changed very little from the early fusion experiments. The vast majority of antibodies used in diagnosis and research, as well as most of the antibodies currently undergoing therapeutic trials, have been made by ‘conventional’ somatic cell hybrids between spleen cells from immunized mice and murine myeloma cells. In parallel, ‘in the wings’, a whole new technology has been developing. In this new technology, which may loosely be called antibody engineering, immunoglobulin gene segments are manipulated in vitro to produce proteins that combine the specificity of the immunoglobulin molecule with a variety of useful properties.
The field of antibody engineering exists to make more useful antibodies, and there is, in principle, no barrier between those scientists whose primary aim is to make useful antibodies and those who are specialized in antibody engineering technology. Yet there is some justification for the statement that, to a significant extent, researchers with a primary interest in the uses of monoclonal antibodies have stuck to the conventional hybridoma technology, whilst the pioneers of antibody engineering have been so occupied with developing the technology that they have not taken their products through to their eventual applications. This is not to say that anyone has lost sight of the applications, rather that the choice between using a technology that has not yet reached its ultimate potential and continuing to improve the technology is always a difficult one.
The major focus of this book is to examine the several aspects of antibody engineering, and to assess whether, when and how the new technology will replace conventional hybridoma technology as a source of useful reagents. The book brings together topics and authors from conventional hybridoma technology and antibody engineering, and the goal is to bring together readers from the two fields, in order to remove any barriers that do exist and expedite the applications of improved, engineered antibodies.
2. Monoclonal antibodies: scope and limitations
Monoclonal antibodies have become so much a part of biology and medicine that a catalog of their applications seems superfluous. It is more useful to consider areas in which monoclonal antibodies have had a limited impact, in order to focus our thoughts on possible improvements.
The relevant properties of antibodies
Figure 1 illustrates schematically the major potential applications of antibodies. These applications are all based on the same fundamental property of the antibody molecule, the ability to bind specifically, or selectively, to a particular molecular structure. In some of the applications, other properties of the antibody molecule, such as the ability to bind complement and lyse target cells, are used. However, for most applications of antibodies, the only property of the antibody molecule that is useful is its antigen-binding capacity. Indeed, the other properties of the molecule may be a nuisance, and this is part of the reason why antibody engineering is potentially useful. Figure 2 illustrates schematically the prototypic immunoglobulin molecule, and relates its major properties to the relevant structural features.
The ability, in quantitative terms, of the antibody molecule to bind antigen and its specificity for that antigen are not necessarily the same property, and the relative importance of these two facets of antigen binding will depend on the application. Specificity, the ability to distinguish a particular molecular structure from all other structures, is clearly important when an antibody is being used to identify, measure or purify a component of a mixture, or to target particular cells in vivo. Realistically, antibody is never absolutely specific; we cannot know that it distinguishes its target structure from all other structures. We need to know that it is specific within the context in which it is being used, that it distinguishes its target structure from all the potential interfering structures in the mixture being analyzed.

Figure 1. The major applications of antibodies.
Once adequate operational specificity is assured, the strength of binding may be the limiting factor. By binding strength, we mean either the affinity of interaction between antibody and antigen, or just the number of antibody molecules bound to the antigen.
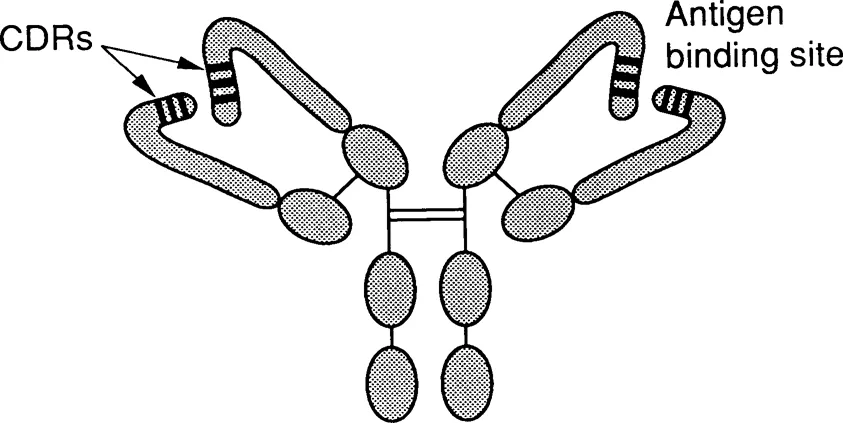
Figure 2. The antibody molecule. Antibodies exist in a variety of oligomeric forms; the figure illustrates a typical IgG molecule.
Monoclonal antibody and polyclonal antibody preparations
The relative importance of specificity and binding strength in different applications explains, in part, why there are applications where monoclonal antibodies have not replaced polyclonal antibody preparations. The major difference between monoclonal antibody and polyclonal preparations is that the latter are always mixtures of many antibodies. This means that monoclonal antibodies are more specific, because amongst the polyclonal mixture will be some antibodies that react with other structures. On the other hand, because a polyclonal preparation will contain multiple antibodies reacting with different parts of the target molecule or cell, polyclonal antibodies almost invariably give a stronger reaction (more antibody bound) than monoclonal antibody. Furthermore, the multiplicity of antibodies favors polyclonal preparations in assays that depend on the formation of large antigen—antibody aggregates (discussed in more detail later in this chapter). If the lower specificity of the polyclonal preparation is acceptable, it will be preferred over monoclonal antibody.
There is, however, another major difference between conventional polyclonal anti sera and monoclonal antibodies: the reproducibility of the latter. Once a hybridoma is established, with reasonable care and quality control it should be possible to produce identical batches of material forever. Antisera, on the other hand, will vary from animal to animal, and from batch to batch when taken from the same animal. A reasonable conclusion from this is that immunoassays, and other applications where monoclonal antibodies have not displaced polyclonal preparations, would benefit from the use of monoclonal antibodies, provided binding could be increased. One possible approach to achieving this is to make mixtures of monoclonal antibodies, against different epitopes. This has been discussed many times, but there are few examples of practical application. In the case of antibody-binding assays, where the amount of antibody bound may be limiting, another approach is to improve signal detection, so that an adequate signal can be obtained from a single monoclonal antibody. In effect this has been done for the analysis of membrane markers on lymphocytes in suspension and in tissue sections, where monoclonal antibodies have very largely replaced polyclonal sera.
Polyclonal sera are still utilized in many immunoassays. A brief examination of a routine diagnostic immunology laboratory will reveal that, whilst monoclonal antibodies are used exclusively (other than the anti-mouse detection reagent) for cellular phenotyping, most of the other immunoassays utilize polyclonal sera. Many of these assays involve precipitation of antigen—antibody complexes, either in gel or in suspension (turbidimetric or nephelometric techniques), or aggregation of cells or particles (agglutination). Polyclonal antisera tend to be more effective in precipitation techniques, which depend on the formation of large aggregates of antigen and antibody. In a polyclonal antibody mixture, there will be antibodies against several different epitopes on the antigen molecule, leading to more effective cross-linking of molecules and consequent precipitation. On the other hand, enzyme-linked immunosorbent assays (ELISAs), which do not depend on precipitation, are increasingly based on monoclonal reagents. Many of the assays based on polyclonal reagents work well and are adequately quality controlled, so there is little incentive to change. However, the more specificity is required the more difficult it is to achieve consistent results with polyclonal antibodies. The ultimate answer will be to make controlled mixtures of monoclonal antibodies against different epitopes.
Polyclonal anti-mouse Ig
Perhaps the only growth area for polyclonal antisera since the establishment of monoclonal antibodies is, paradoxically, in the market for anti-mouse Ig antibodies to use in conjunction with mouse monoclonal antibodies. A typical application is indirect immunofluorescence (Figure 3), where a monoclonal antibody binds specifically to a cell membrane marker and is detected by fluorescein-conjugated antibody (made in rabbit, sheep, etc.) against murine Ig. The fluorescent dye can be conjugated directly to the monoclonal antibody (Figure 4), and there are advantages and disadvantages to both approaches. The major advantage of indirect immunofluorescence (the same would apply to assays where the reporter molecule is an enzyme or a radioisotope) is that a single detection reagent can be used in conjunction with many monoclonal antibodies. A secondary advantage is that indirect techniques tend to be more sensitive, because each layer in a multi-step staining sequence amplifies the signal. The major disadvantage of indirect techniques is the relatively lower specificity of polyclonal antibodies as reagents. For example, even a good sheep anti-mouse Ig will cross-react to some extent with hu...
Table of contents
- Cover
- Half Title
- Title Page
- Copyright Page
- Table of Contents
- Contributors
- Abbreviations
- Preface
- 1. Introduction.
- 2. Making and using ‘conventional’ mouse monoclonal antibodies.
- 3. Human monoclonal antibodies from immortalized B cells.
- 4. Reconstruction of monoclonal antibodies by genetic engineering.
- 5. The genetic engineering of antibody constructs for diagnosis and therapy.
- 6. Superseding hybridoma technology with phage display libraries.
- 7. Construction and application of libraries of artificial antibodies.
- 8. Expression of antibody genes in mammalian cells.
- 9. Structure and application of single-chain Fvs as diagnostic and therapeutic agents.
- Index