
eBook - ePub
Monoclonal Antibodies
The Second Generation
Heddy Zola, Heddy Zola
This is a test
Partager le livre
- 220 pages
- English
- ePUB (adapté aux mobiles)
- Disponible sur iOS et Android
eBook - ePub
Monoclonal Antibodies
The Second Generation
Heddy Zola, Heddy Zola
Détails du livre
Aperçu du livre
Table des matières
Citations
À propos de ce livre
A review of what needs to be done to realise the potential of monoclonal antibodies. The book assesses the competing technologies with advice on the best approach for a particular situation.
Foire aux questions
Comment puis-je résilier mon abonnement ?
Il vous suffit de vous rendre dans la section compte dans paramètres et de cliquer sur « Résilier l’abonnement ». C’est aussi simple que cela ! Une fois que vous aurez résilié votre abonnement, il restera actif pour le reste de la période pour laquelle vous avez payé. Découvrez-en plus ici.
Puis-je / comment puis-je télécharger des livres ?
Pour le moment, tous nos livres en format ePub adaptés aux mobiles peuvent être téléchargés via l’application. La plupart de nos PDF sont également disponibles en téléchargement et les autres seront téléchargeables très prochainement. Découvrez-en plus ici.
Quelle est la différence entre les formules tarifaires ?
Les deux abonnements vous donnent un accès complet à la bibliothèque et à toutes les fonctionnalités de Perlego. Les seules différences sont les tarifs ainsi que la période d’abonnement : avec l’abonnement annuel, vous économiserez environ 30 % par rapport à 12 mois d’abonnement mensuel.
Qu’est-ce que Perlego ?
Nous sommes un service d’abonnement à des ouvrages universitaires en ligne, où vous pouvez accéder à toute une bibliothèque pour un prix inférieur à celui d’un seul livre par mois. Avec plus d’un million de livres sur plus de 1 000 sujets, nous avons ce qu’il vous faut ! Découvrez-en plus ici.
Prenez-vous en charge la synthèse vocale ?
Recherchez le symbole Écouter sur votre prochain livre pour voir si vous pouvez l’écouter. L’outil Écouter lit le texte à haute voix pour vous, en surlignant le passage qui est en cours de lecture. Vous pouvez le mettre sur pause, l’accélérer ou le ralentir. Découvrez-en plus ici.
Est-ce que Monoclonal Antibodies est un PDF/ePUB en ligne ?
Oui, vous pouvez accéder à Monoclonal Antibodies par Heddy Zola, Heddy Zola en format PDF et/ou ePUB ainsi qu’à d’autres livres populaires dans Medicine et Immunology. Nous disposons de plus d’un million d’ouvrages à découvrir dans notre catalogue.
Informations
Chapter 1
Introduction
1. Introduction: the purpose of this book
The first monoclonal antibodies were described in 1975 (Kohler and Milstein, 1975, 1976), and the first antibodies made with particular applications in mind followed in 1977–78 (Barnstable et al., 1978; Galfre et al., 1977; White et al., 1978; Williams et al., 1977). The potential use of monoclonal antibodies as reagents was soon appreciated widely, leading to rapid growth in the field. Some laboratories turned their entire resources over to the preparation and characterization of monoclonal antibodies, a new sector of the reagents and biotechnology industry developed around monoclonal antibodies, and many immunoassays were greatly improved by the use of monoclonal antibodies. There are now entire fields of research and diagnosis which would be impossible without monoclonal antibodies (the detailed analysis of leukocyte phenotype (Knapp et al., 1989; McMichael et al., 1987; Schlossman et al., 1994) provides just one example). The therapeutic application of monoclonal antibodies, whilst still limited in scope, promises to break its substantial shackles and realize the potential forecast by its proponents.
Most of this has been achieved using techniques which have changed very little from the early fusion experiments. The vast majority of antibodies used in diagnosis and research, as well as most of the antibodies currently undergoing therapeutic trials, have been made by ‘conventional’ somatic cell hybrids between spleen cells from immunized mice and murine myeloma cells. In parallel, ‘in the wings’, a whole new technology has been developing. In this new technology, which may loosely be called antibody engineering, immunoglobulin gene segments are manipulated in vitro to produce proteins that combine the specificity of the immunoglobulin molecule with a variety of useful properties.
The field of antibody engineering exists to make more useful antibodies, and there is, in principle, no barrier between those scientists whose primary aim is to make useful antibodies and those who are specialized in antibody engineering technology. Yet there is some justification for the statement that, to a significant extent, researchers with a primary interest in the uses of monoclonal antibodies have stuck to the conventional hybridoma technology, whilst the pioneers of antibody engineering have been so occupied with developing the technology that they have not taken their products through to their eventual applications. This is not to say that anyone has lost sight of the applications, rather that the choice between using a technology that has not yet reached its ultimate potential and continuing to improve the technology is always a difficult one.
The major focus of this book is to examine the several aspects of antibody engineering, and to assess whether, when and how the new technology will replace conventional hybridoma technology as a source of useful reagents. The book brings together topics and authors from conventional hybridoma technology and antibody engineering, and the goal is to bring together readers from the two fields, in order to remove any barriers that do exist and expedite the applications of improved, engineered antibodies.
2. Monoclonal antibodies: scope and limitations
Monoclonal antibodies have become so much a part of biology and medicine that a catalog of their applications seems superfluous. It is more useful to consider areas in which monoclonal antibodies have had a limited impact, in order to focus our thoughts on possible improvements.
The relevant properties of antibodies
Figure 1 illustrates schematically the major potential applications of antibodies. These applications are all based on the same fundamental property of the antibody molecule, the ability to bind specifically, or selectively, to a particular molecular structure. In some of the applications, other properties of the antibody molecule, such as the ability to bind complement and lyse target cells, are used. However, for most applications of antibodies, the only property of the antibody molecule that is useful is its antigen-binding capacity. Indeed, the other properties of the molecule may be a nuisance, and this is part of the reason why antibody engineering is potentially useful. Figure 2 illustrates schematically the prototypic immunoglobulin molecule, and relates its major properties to the relevant structural features.
The ability, in quantitative terms, of the antibody molecule to bind antigen and its specificity for that antigen are not necessarily the same property, and the relative importance of these two facets of antigen binding will depend on the application. Specificity, the ability to distinguish a particular molecular structure from all other structures, is clearly important when an antibody is being used to identify, measure or purify a component of a mixture, or to target particular cells in vivo. Realistically, antibody is never absolutely specific; we cannot know that it distinguishes its target structure from all other structures. We need to know that it is specific within the context in which it is being used, that it distinguishes its target structure from all the potential interfering structures in the mixture being analyzed.

Figure 1. The major applications of antibodies.
Once adequate operational specificity is assured, the strength of binding may be the limiting factor. By binding strength, we mean either the affinity of interaction between antibody and antigen, or just the number of antibody molecules bound to the antigen.
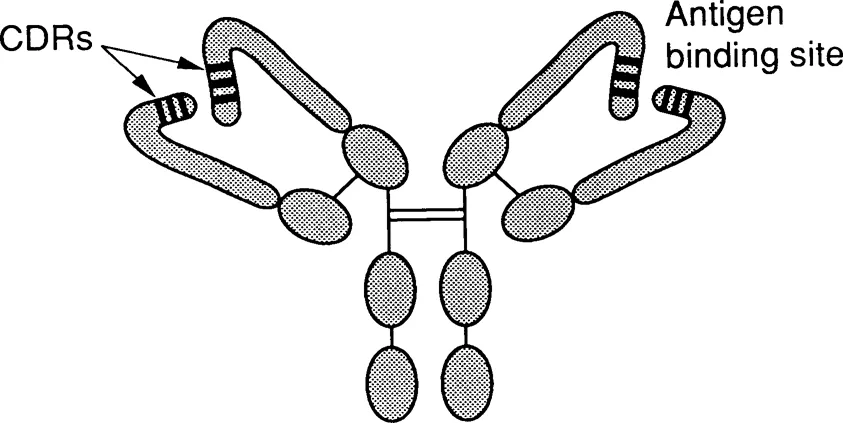
Figure 2. The antibody molecule. Antibodies exist in a variety of oligomeric forms; the figure illustrates a typical IgG molecule.
Monoclonal antibody and polyclonal antibody preparations
The relative importance of specificity and binding strength in different applications explains, in part, why there are applications where monoclonal antibodies have not replaced polyclonal antibody preparations. The major difference between monoclonal antibody and polyclonal preparations is that the latter are always mixtures of many antibodies. This means that monoclonal antibodies are more specific, because amongst the polyclonal mixture will be some antibodies that react with other structures. On the other hand, because a polyclonal preparation will contain multiple antibodies reacting with different parts of the target molecule or cell, polyclonal antibodies almost invariably give a stronger reaction (more antibody bound) than monoclonal antibody. Furthermore, the multiplicity of antibodies favors polyclonal preparations in assays that depend on the formation of large antigen—antibody aggregates (discussed in more detail later in this chapter). If the lower specificity of the polyclonal preparation is acceptable, it will be preferred over monoclonal antibody.
There is, however, another major difference between conventional polyclonal anti sera and monoclonal antibodies: the reproducibility of the latter. Once a hybridoma is established, with reasonable care and quality control it should be possible to produce identical batches of material forever. Antisera, on the other hand, will vary from animal to animal, and from batch to batch when taken from the same animal. A reasonable conclusion from this is that immunoassays, and other applications where monoclonal antibodies have not displaced polyclonal preparations, would benefit from the use of monoclonal antibodies, provided binding could be increased. One possible approach to achieving this is to make mixtures of monoclonal antibodies, against different epitopes. This has been discussed many times, but there are few examples of practical application. In the case of antibody-binding assays, where the amount of antibody bound may be limiting, another approach is to improve signal detection, so that an adequate signal can be obtained from a single monoclonal antibody. In effect this has been done for the analysis of membrane markers on lymphocytes in suspension and in tissue sections, where monoclonal antibodies have very largely replaced polyclonal sera.
Polyclonal sera are still utilized in many immunoassays. A brief examination of a routine diagnostic immunology laboratory will reveal that, whilst monoclonal antibodies are used exclusively (other than the anti-mouse detection reagent) for cellular phenotyping, most of the other immunoassays utilize polyclonal sera. Many of these assays involve precipitation of antigen—antibody complexes, either in gel or in suspension (turbidimetric or nephelometric techniques), or aggregation of cells or particles (agglutination). Polyclonal antisera tend to be more effective in precipitation techniques, which depend on the formation of large aggregates of antigen and antibody. In a polyclonal antibody mixture, there will be antibodies against several different epitopes on the antigen molecule, leading to more effective cross-linking of molecules and consequent precipitation. On the other hand, enzyme-linked immunosorbent assays (ELISAs), which do not depend on precipitation, are increasingly based on monoclonal reagents. Many of the assays based on polyclonal reagents work well and are adequately quality controlled, so there is little incentive to change. However, the more specificity is required the more difficult it is to achieve consistent results with polyclonal antibodies. The ultimate answer will be to make controlled mixtures of monoclonal antibodies against different epitopes.
Polyclonal anti-mouse Ig
Perhaps the only growth area for polyclonal antisera since the establishment of monoclonal antibodies is, paradoxically, in the market for anti-mouse Ig antibodies to use in conjunction with mouse monoclonal antibodies. A typical application is indirect immunofluorescence (Figure 3), where a monoclonal antibody binds specifically to a cell membrane marker and is detected by fluorescein-conjugated antibody (made in rabbit, sheep, etc.) against murine Ig. The fluorescent dye can be conjugated directly to the monoclonal antibody (Figure 4), and there are advantages and disadvantages to both approaches. The major advantage of indirect immunofluorescence (the same would apply to assays where the reporter molecule is an enzyme or a radioisotope) is that a single detection reagent can be used in conjunction with many monoclonal antibodies. A secondary advantage is that indirect techniques tend to be more sensitive, because each layer in a multi-step staining sequence amplifies the signal. The major disadvantage of indirect techniques is the relatively lower specificity of polyclonal antibodies as reagents. For example, even a good sheep anti-mouse Ig will cross-react to some extent with hu...