
- 232 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
About this book
Chemical Projects Scale Up: How to Go from Laboratory to Commercial covers the chemical engineering steps necessary for taking a laboratory development into the commercial world. The book includes the problems associated with scale up, equipment sizing considerations, thermal characteristics associated with scale up, safety areas to consider, recycling considerations, operability reviews and economic viability. In addition to the process design aspects of commercializing the laboratory development, consideration is given to the utilization of a development in an existing plant.- Explains how heat removal for exothermic reactions can be scaled up- Outlines how a reactor can be sized from batch kinetic data- Discusses how the plant performance of a new catalyst can be evaluated- Presents how the economics of a new product/process can be developed- Discusses the necessary evaluation of recycling in commercial plants
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Information
Potential Problems With Scale-up
Abstract
Keywords
- • Equipment Related—Scale-up will always involve larger equipment and possibly a change in equipment design such as a jacketed vessel to a pump-around exchanger loop.
- • Mode Related—A typical scale-up may well involve a change in equipment mode such as a change from a batch reactor to a continuous reactor.
- • Theoretical Considerations—Although theoretical conclusions and considerations have generally been the regime of the laboratory chemists, the scale-up should consider if it is possible that anything is missed or erroneous conclusions have been drawn.
- • Thermal Characteristics—Scale-up of a reactor or any other vessel where heat transfer is occurring will always require consideration due to the potentially reduced area-to-volume ratio.
- • Safety Considerations—Although safety is always a consideration, it is more so as the equipment size is increased.
- • Recycle Considerations—Several factors require that recycle facilities be included in commercial facilities, although it may not be as important in laboratory or pilot plant facilities.
- • Regulatory Requirements—Regulation might include products, process, and by-product disposal.
- • Project Focus Considerations—The project team must maintain focus on the development and the economic aspects of the process.
Equipment Related
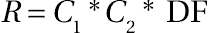
- where
- R = The kinetic rate of the process.
- C1 = A constant determined in the laboratory, pilot plant, or a smaller commercial plant. Generally, this constant will not change as the process is scaled up to the larger rate.
- C2 = A constant that is related to the equipment’s physical attributes such as area, volume, L/D ratio, mixer speed, mixer design, exchanger design, or any other physical attribute. This constant will be a strong function of the scale-up ratio. As discussed later, it is not always identical to the scale-up ratio.
- DF = The driving force that relates to a difference between an equilibrium value and an actual value. This might be temperature driving force, reactant concentration driving force, or volatiles concentration driving force. The driving force will always have the form of the actual value minus an equilibrium value.
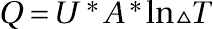
- where
- Q = The kinetic rate or, in the case of heat transfer, it is the rate of heat removal/addition expressed in heat units (BTU or calories) per unit time (normally hours).
- U = The constant comparable to C1 or, in this case, the heat transfer coefficient.
- A = The heat transfer area, which is the constant comparable to C2.
- ln∆T = The temperature driving force or, in this case, the “log delta T.”
- • What is the required ratio of the kinetic value R? This maybe the ratio of the production rates between the smaller facilities and the larger facility. However, when considering the example of a heat exchanger, the ratio of heat exchanged (Q) may be more or less than the production rate ratio, because the inlet and outlet temperatures may be different in a commercial unit than a bench scale or a pilot plant. Another possibility to be considered is a jacketed reactor with a pump-around cooling loop. In such a scenario, due to the reduced area-to-volume ratio (A/V) in the jacketed reactor of the larger unit, the actual Q on the external pump-around heat exchanger maybe higher than that which would be calculated by a direct ratio of total heat exchanged in the two reactors. The key concept is that the actual scale-up should be based on the unit operation of interest rather than a simple ratio of production rates.
- • Is it really reasonable to assume that C1 does not change between the smaller and larger facility? Generally, the answer is that this is a reasonable assumption. However, when considering problems with scale-up, this is an area that must be considered. A real-life example of the change of C1 was a vertical heat exchanger in a commercial plant being used to condense propylene. This exchanger was scaled up to a higher capacity while maintaining C1 (the heat transfer coefficient, U) constant at the value demonstrated in the smaller facility. During ...
Table of contents
- Cover
- Title page
- Table of Contents
- Copyright
- Introduction
- 1: Potential Problems With Scale-up
- 2: Equipment Design Considerations
- 3: Developing Commercial Process Flow Sheets
- 4: Thermal Characteristics for Reactor Scale-up
- 5: Safety Considerations
- 6: Recycle Considerations
- 7: Supplier’s Equipment Scale-up
- 8: Sustainability
- 9: Project Evaluation Using CAPEX and OPEX Inputs
- 10: Emerging Technology Contingency (ETC)
- 11: Other Uses of Study Designs
- 12: Scaling Up to Larger Commercial Sizes
- 13: Defining and Mitigating Risks
- 14: Typical Cases Studies
- Epilogue: Final Words and Acknowledgments
- Index