
Clinical Trials
Study Design, Endpoints and Biomarkers, Drug Safety, and FDA and ICH Guidelines
- 896 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Clinical Trials
Study Design, Endpoints and Biomarkers, Drug Safety, and FDA and ICH Guidelines
About this book
Clinical Trials, Second Edition, offers those engaged in clinical trial design a valuable and practical guide. This book takes an integrated approach to incorporate biomedical science, laboratory data of human study, endpoint specification, legal and regulatory aspects and much more with the fundamentals of clinical trial design. It provides an overview of the design options along with the specific details of trial design and offers guidance on how to make appropriate choices. Full of numerous examples and now containing actual decisions from FDA reviewers to better inform trial design, the 2nd edition of Clinical Trials is a must-have resource for early and mid-career researchers and clinicians who design and conduct clinical trials.- Contains new and fully revised material on key topics such as biostatistics, biomarkers, orphan drugs, biosimilars, drug regulations in Europe, drug safety, regulatory approval and more- Extensively covers the "study schema" and related features of study design- Incorporates laboratory data from studies on human patients to provide a concrete tool for understanding the concepts in the design and conduct of clinical trials- Includes decisions made by FDA reviewers when granting approval of a drug as real world learning examples for readers
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
Origins of Drugs
Abstract
Keywords
I Introduction
II Structures of Drugs
a Origin of Warfarin
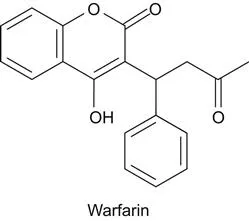
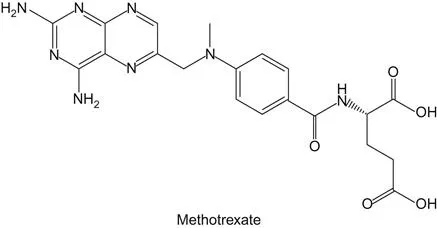
b Origins of Methotrexate and 5-Fluorouracil
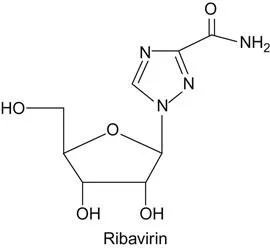
c Origin of Ribavirin
d Origin of Paclitaxel
Table of contents
- Cover image
- Title page
- Table of Contents
- Copyright
- Dedication
- Acknowledgments
- Preface
- Introduction
- Abbreviations and Definitions
- Biographies
- Chapter 1. Origins of Drugs
- Chapter 2. Clinical Trial Design
- Chapter 3. Run-In Period
- Chapter 4. Inclusion/Exclusion Criteria, Stratification, and Subgroups—Part I
- Chapter 5. Inclusion/Exclusion Criteria, Stratification, and Subgroups—Part II
- Chapter 6. Blinding, Randomization, and Allocation
- Chapter 7. Placebo Arm as Part of Clinical Trial Design
- Chapter 8. Intent-to-Treat Analysis Versus Per Protocol Analysis
- Chapter 9. Biostatistics—Part I
- Chapter 10. Biostatistics—Part II
- Chapter 11. Introduction to Endpoints
- Chapter 12. Oncology Endpoint—Objective Response
- Chapter 13. Oncology Endpoints: Overall Survival and Progression-Free Survival
- Chapter 14. Oncology Endpoints: Time to Progression
- Chapter 15. Oncology Endpoint: Disease-Free Survival
- Chapter 16. Oncology Endpoint: Time to Distant Metastasis
- Chapter 17. Neoadjuvant Therapy Versus Adjuvant Therapy
- Chapter 18. Hematological Cancers
- Chapter 19. Biomarkers
- Chapter 20. Endpoints for Immune Diseases
- Chapter 21. Endpoints for Infections
- Chapter 22. Health-Related Quality of Life Tools—Oncology
- Chapter 23. Health-Related Quality-of-Life Tools—Immune Disorders
- Chapter 24. Health-Related Quality-of-Life Tools—Infections
- Chapter 25. Drug Safety
- Chapter 26. Mechanism of Action of Diseases and Drugs—Part I
- Chapter 27. Mechanism of Action—Part II (Cancer)
- Chapter 28. Mechanism of Action—Part III (Immune Disorders)
- Chapter 29. Mechanisms of Action—Part IV (Infections)
- Chapter 30. Consent Forms
- Chapter 31. Package Inserts
- Chapter 32. Warning Letters
- Chapter 33. Regulatory Approval
- Chapter 34. Patents
- Index
- Sync with Jellybooks