
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Biomarkers in Cancer Screening and Early Detection
About this book
Prepared by world leaders on this topic, Biomarkers in Cancer Screening and Early Detection offers a comprehensive, state-of-the-art perspective on the various research and clinical aspects of cancer biomarkers, from their discovery and development to their validation, clinical utility, and use in developing personalized cancer treatment.
- Offers a comprehensive, state-of-the-art perspective on the various research and clinical aspects of cancer biomarkers
- Provides immediately actionable information – and hopefully also inspiration – to move discovery and clinical application forward
- Offers vital knowledge to help develop personalized cancer treatment for individual patients with specific cancers
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Biomarkers in Cancer Screening and Early Detection by Sudhir Srivastava in PDF and/or ePUB format, as well as other popular books in Medicine & Oncology. We have over one million books available in our catalogue for you to explore.
Information
Part I
Foundations of Biomarker Research
Chapter 1
Nuts and Bolts of Biomarker Research
Sharmistha Ghosh1 and Sudhir Srivastava2
1Cancer Biomarkers Research Group, Division of Cancer Prevention, National Cancer Institute, Rockville, MD, USA
2Cancer Biomarkers Research Group, Division of Cancer Prevention, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA
What is a biomarker?
A biomarker is a particular characteristic, or a molecular fingerprint, which indicates manifestation of a physiological state, and which can be objectively quantified to distinguish a normal state from a pathological condition (e.g., cancer) or a response to a therapeutic intervention. The National Cancer Institute (NCI) of the National Institutes of Health (NIH) defines biomarker as: “A biological molecule found in blood, other body fluids, or tissues that is a sign of a normal or abnormal process, or of a condition or disease. A biomarker may be used to see how well the body responds to a treatment for a disease or condition. Biomarkers are also called molecular markers and signature molecules.” [1]
As a normal cell undergoes a complex process of transformation into a cancerous state, it is hoped that measurable characteristics can be analyzed to derive a meaningful clinical decision – either directly, from the early-stage tumor before it is palpable or detectable by sensitive screening technologies available at this time, or as a result of an immunological response to the tumor. The characteristics include a broad range of biochemical entities such as nucleic acids (e.g., DNA, mRNA, long and small [short] non-coding RNA), proteins, post-translationally modified proteins (e.g., phosphoproteins, glycoproteins, methylated proteins, glycolipids), sugars, lipids and small metabolites, as well as whole circulating cells or biophysical characteristics of tissues.
Failure to detect an identifiable molecular mar-ker may not be a negative predictor of malignancy, and a positive test for a molecular marker may not always be a positive predictor of malignancy. However, an ideal biomarker should indicate a reliable positive or negative correlation with the presence of the disease, which means that the clinical test for the biomarker should have high sensitivity (true positive rate – that is, the ability to correctly identify individuals with the disease) and specificity (true negative rate – that is, the ability to correctly identify individuals without the disease). The clinical value of a biomarker test is based on its positive predictive value (PPV), or how likely it is for test-positive individuals to actually have the disease, and its negative predictive value (NPV), or how likely it is for test-negative individuals to not have the disease. These again depend on the prevalence of the disease in the population of interest. Biomarkers also need to be easily accessible (e.g., by non-invasive methods for screening purposes), quantifiable, analyzable, and interpretable.
Why biomarker research is imperative
The development of cancer is preceded by numerous germline and somatic mutations, structural changes in chromosomes, and other genetic and epigenetic changes, which transform normal cells into benign tumors and, progressively, into malignant and metastatic forms. Cancer is a heterogeneous, multigenic group of diseases; the heterogeneity lies not only at the biochemical level (genes, proteins, metabolites), but also at the tissue and population level (e.g., [2–10]). The enormous complexity makes cancer detection, diagnosis, and treatment quite challenging. Although cancers diagnosed at earlier stages have a much better prognosis compared with cancers diagnosed at later stages, it is noteworthy that many cancer patients are diagnosed at a stage at which the cancer is too far advanced to be cured.
Currently, recommendations for early detection of cancer in average-risk individuals are available for colorectal, cervical, breast, endometrial (in menopausal women) and prostate cancers, and in high-risk individuals in the case of lung cancer. There has been a substantial increase in “cancer” incidence as a result of screening, but without a proportional decrease in mortality despite treatment. This implies that screening identifies a large reservoir of indolent cancers (overdiagnosis) [11], which would have never become symptomatic without screening, and did not require any treatment. However, because it is not known at this time which lesions are indolent, many individuals are put through intensive treatments unnecessarily, which often causes anxiety as well as substantial physical and financial harm. An extensive discussion on existing screening modalities, recommendations, and the consequences and complexities involved is beyond the scope of this chapter. A shared decision-making discussion between the patients and their physicians, based on existing data, and also taking into consideration an individual patient's values and philosophies on healthcare, is important [12].
The ability to identify tumors that are destined to progress, and which are associated with morbidity and mortality at an early stage, will allow effective treatment interventions and reduce deaths. Identification of tumor-specific molecular signatures is imperative for a new approach to early detection, diagnosis, prognosis, disease classification and risk prediction. It will also help to implement appropriate treatment decisions and therapeutic interventions, to monitor treatment response and efficacy (i.e., a measurable effect on a clinical end point), and to overcome drug resistance in a precise, patient-specific approach. Such practice of tailored “personalized medicine”, based on the molecular portraits of tumor cells, allows physicians to inform individual patients of the expected outcomes – for example, whether treatments or surveillance approaches will be beneficial, and when to stop treatment based on response to drug(s). An illustration of several windows of clinical relevance in the management of cancer during its course of development is shown where different biomarker profiles can be applied to each of these windows for optimal management of cancer (Figure 1.1).
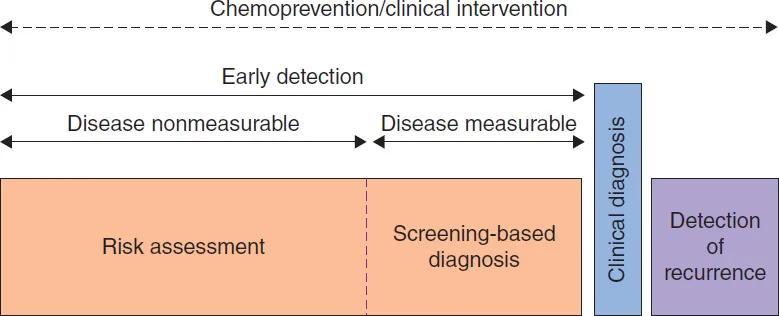
Figure 1.1 Biomarker application in the clinic. A long window of opportunity for chemoprevention or any clinical intervention is divided into sub-windows, based on whether a risk assessment is made when the disease is non-measureable, or an early diagnosis is made based on screening for measurable characteristics of the tumor, or a clinical diagnosis is made when the disease is symptomatic or has recurred. Adapted from [13].
A few biomarkers discussed in this section underscore their utilization as clinical tools for facilitating diagnosis and treatment of tumors. Germline mutations in the high-penetrance genes breast cancer 1 (BRCA1) and breast cancer 2 (BRCA2), which are associated with hereditary susceptibility to breast and ovarian cancers, somatic mutations in phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA) gene in colorectal tumors which can act as predictive biomarker for adjuvant aspirin therapy, and metastatic melanoma patients who harbor v...
Table of contents
- Cover
- Series
- Title Page
- Copyright
- Dedication
- List of Contributors
- Preface
- Part I: Foundations of Biomarker Research
- Part II: State-of-the-Science in Organ-Specific Biomarker Research
- Part III: Biomarkers, Screening and Precision Health: Implications for Public Health
- Index
- EULA