
- 494 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
The Biodiesel Handbook
About this book
The second edition of this invaluable handbook covers converting vegetable oils, animal fats, and used oils into biodiesel fuel. The Biodiesel Handbook delivers solutions to issues associated with biodiesel feedstocks, production issues, quality control, viscosity, stability, applications, emissions, and other environmental impacts, as well as the status of the biodiesel industry worldwide.- Incorporates the major research and other developments in the world of biodiesel in a comprehensive and practical format- Includes reference materials and tables on biodiesel standards, unit conversions, and technical details in four appendices- Presents details on other uses of biodiesel and other alternative diesel fuels from oils and fats
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
Introduction
What Is Biodiesel?
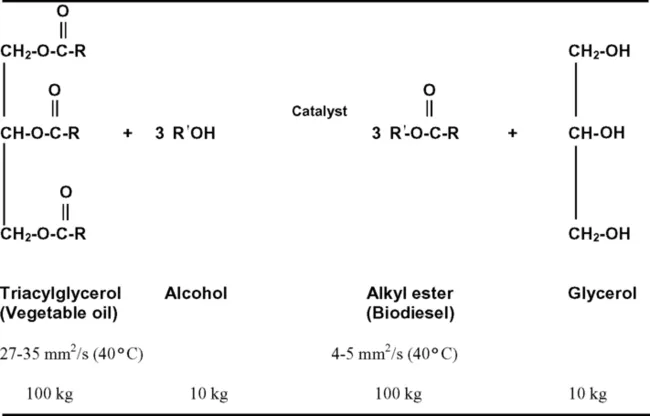
Why are Vegetable Oils and Animal Fats Transesterified to Alkyl Esters (Biodiesel)?
Why Can Vegetable Oils, Animal Fats, and Their Derivatives be Used as (Alternative) Diesel Fuel?
History of Vegetable Oil-Based Diesel Fuels
Rudolf Diesel
Table of contents
- Cover image
- Title page
- Table of Contents
- Copyright
- Preface to the Second Edition
- Preface to the First Edition
- Contributing Authors
- Chapter 1: Introduction
- Chapter 2: History of Vegetable Oil-Based Diesel Fuels
- Chapter 3: Basics of Diesel Engines and Diesel Fuels
- Chapter 4: Biodiesel Production
- Chapter 5: Analytical Methods
- Chapter 6: Fuel Properties
- Chapter 7: Exhaust Emissions
- Chapter 8: Current Status of the Biodiesel Industry
- Chapter 9: Other Uses of Biodiesel
- Chapter 10: Other Alternative Diesel Fuels from Vegetable Oils and Animal Fats
- Chapter 11: Glycerol Technology Options for Biodiesel Industry
- Appendix A: Technical Tables
- Appendix B: Biodiesel Standards
- Appendix C: Unit Conversions
- Appendix D: Internet Resources
- Index
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app