
eBook - ePub
Surface Engineering of Light Alloys
Aluminium, Magnesium and Titanium Alloys
- 680 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
About this book
The growing use of light alloys in industries such as aerospace, sports equipment and biomedical devices is driving research into surface engineering technologies to enhance their properties for the desired end use. Surface engineering of light alloys: Aluminium, magnesium and titanium alloys provides a comprehensive review of the latest technologies for modifying the surfaces of light alloys to improve their corrosion, wear and tribological properties.Part one discusses surface degradation of light alloys with chapters on corrosion behaviour of magnesium alloys and protection techniques, wear properties of aluminium-based alloys and tribological behaviour of titanium alloys. Part two reviews surface engineering technologies for light alloys including anodising, plasma electrolytic oxidation, thermal spraying, cold spraying, physical vapour deposition, plasma assisted surface treatment, PIII/PSII treatments, laser surface modification, ceramic conversion and duplex treatments. Part three covers applications for surface engineered light alloys including sports equipment, biomedical devices and plasma electrolytic oxidation and anodised aluminium alloys for spacecraft applications.With its distinguished editor and international team of contributors, Surface engineering of light alloys: Aluminium, magnesium and titanium alloys is a standard reference for engineers, metallurgists and materials scientists looking for a comprehensive source of information on surface engineering of aluminium, magnesium and titanium alloys.
- Discusses surface degradation of light alloys considering corrosion behaviour and wear and tribological properties
- Examines surface engineering technologies and modification featuring plasma electrolytic oxidation treatments and both thermal and cold spraying
- Reviews applications for engineered light alloys in sports equipment, biomedical devices and spacecraft
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Surface Engineering of Light Alloys by Hanshan Dong in PDF and/or ePUB format, as well as other popular books in Tecnologia e ingegneria & Ingegneria mineraria. We have over one million books available in our catalogue for you to explore.
Information
Part I
Surface degradation of light alloys
1
Corrosion behavior of magnesium alloys and protection techniques
G.-L. Song, General Motors Corporation, USA
Abstract:
Magnesium alloys have low corrosion resistance and exhibit unusual corrosion behavior in aqueous environments. This chapter briefly summarizes the corrosion characteristics of Mg alloys, such as hydrogen evolution, surface alkalization, macro-galvanic damage and micro-galvanic effect, etc. It also discusses the influences of chemical composition of matrix, secondary phase, and impurities, etc. on the corrosion performance of Mg alloys. Following that, a potential strategy is presented to mitigate their corrosion.
Key words
magnesium
corrosion
protection
1.1 Introduction
Magnesium and its alloys have a high strength/density ratio and have found many successful applications, particularly in the automotive and aerospace industries (Song, 2005b, 2006; Makar and Kruger, 1993; Aghion and Bronfin, 2000; Polmear, 1996; Bettles et al., 2003a and b). However, the poor corrosion resistance of existing magnesium alloys in some service environments has limited the further expansion of their application. Existing investigations have clearly suggested that the corrosion of Mg alloys is unusual in terms of their corrosion behavior (Song, 2004a, 2005b, 2006, 2009a, 2009c; Song and Atrens, 2007; Winzer et al., 2005, 2007, 2008; Wan et al., 2006; Wang et al., 2007). For more successful application of Mg alloys, it is important to understand their characteristic corrosion phenomena. This chapter systematically summarizes the corrosion characteristics of Mg alloys in order to better understand their corrosion performance. Based on this, a potential strategy is proposed to mitigate their corrosion.
1.2 Corrosion behavior of magnesium (Mg) alloys
Mg alloys follow a corrosion mechanism different from other engineering metallic materials such as steel, aluminum alloys, and zinc. on pure Mg or a Mg alloy, it is well known that the overall corrosion reaction can be written as (Makar and Kruger, 1993; Song and Atrens, 1999; Song, 2005b, 2006):
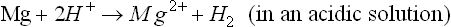
or
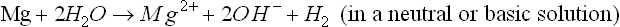
This overall corrosion can be decomposed into anodic and cathodic reactions. The cathodic process is (Song, 2005b, 2006):
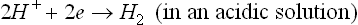
or
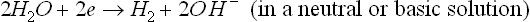
and the anodic process (Song, 2005b, 2006):
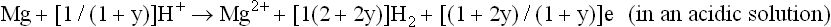
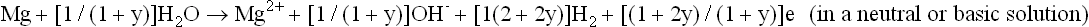
where y is the ratio of Mg + turning into Mg2 + over reacting with H2O to produce H2.
The detailed anodic and cathodic reactions under a steady corrosion condition have been illustrated previously (Song et al., 1997a, 1997b; Song, 2005b; Song and Atrens, 1999, 2003). In general, the anodic dissolution occurs mainly in a film-free area, while in a film covered area the anodic dissolution is negligible. The cathodic reaction, which is mainly a hydrogen evolution process, can take place in both film-free and film-covered areas, but the reaction rate in a film-free area is much faster than the rate in a film-covered area, particularly if impurity particles are present there.
If Mg is cathodically polarized to a very negative potential, Mg is fully covered by a thin film and there is no film-free area. Thus, the anodic dissolution of magnesium is very low, almost zero. However, the cathodic hydrogen evolution can still occur on the thin film surface at such a negative potential. The hydrogen evolution rate will decrease as the polarization potential becomes more positive until a critical potential Ept is reached. At Ept, the surface film starts to break down and both the hydrogen evolution and the Mg anodic dissolution become easier. The dissolution of Mg produces intermediate Mg+ which subsequently leads to generation of hydrogen. In other words, there are two mechanisms of hydrogen evolution; one is a normal cathodic hydrogen evolution process driven by negative polarization potentials, and the other is a magnesium dissolution induced reaction. The process is schematically illustrated in Fig. 1.1.
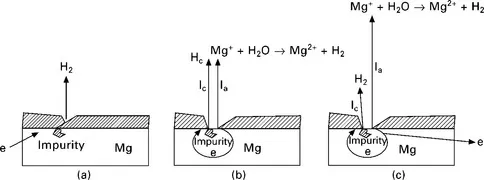
1.1 Electrochemical corrosion and negative difference effect on the magnesium surface (Song, 2005a): (a) at a very negative cathodic potential; (b) at the critical potential Ept; (c) at a potential more positive than Ept.
The reactions involved in the process are as follows:
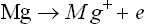
then:
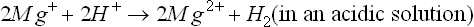
or
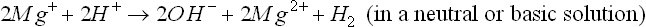
The anodic dissolution rate of Mg (Eq. 1.7) increases as the polarization potential or current becomes more positive. This produces additional hydrogen at a more positive potential via the reactions in Equations 1.8 or 1.9. The hydrogen evolution occurring in a corroding (film-free) area is termed ‘anodic hydrogen evolution’ (AHE), because it is closely associated with the anodic dissolution process. One important aspect is that it becomes faster as the anodic polarization potential becomes more positive, as if it were an anodic process (see Fig. 1.2). This differs from the normal cathodic hydrogen evolution (CHE) in that hydrogen evolution can occur on either the film-free or the film-covered areas, and decreases with positively shifting polarization potential in the cathodic region (see Fig. 1.2).
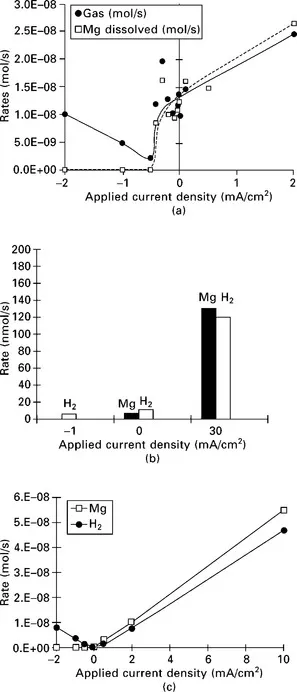
1.2 Hydrogen evolution and Mg dissolution rates: (a) Mg in 1N NaCI (pH 11) (Song et al., 1997b); (b) Mg in 1N Na2SO4 (pH 11) (Song et al., 1997a); (c) AZ21 (matrix phase) in 1N NaCl (pH 11) (Song, 2005b); (d) AZ91 ingot in 1N NaCl (pH 11) (Song, 2005b); (e) diecast AZ91 in 1N NaCl (pH 11) (Song, 2005b); (f) sand cast MEZ in 5 wt% NaCl (Song, 2005b).
1.2.1 Hydrogen evolution
The overall corrosion Equations 1.1. or 1.2 suggest that the dissolution of Mg is always accompanied by hydrogen evolution. This corrosion-related hydrogen evolution is also applicable to Mg alloys in aqueous solutions (Song et al., 1998, 2001, 2005a; Song and Atrens, 1998, 2003; Song and St John, 2002), including engine coolants (Song and St John, 2004, 2005) and simulated body fluids (SBF) (Song and Song, 2006, 2007; Song, 2007b). In a severe corrosion process, Mg particle undermining (Mg particles falling into solution due to the surrounding material being completely corroded) may take place.
However, this process has been shown to have no influence upon either of the reactions in Equations 1.1 or 1.2 (Song et al., 1997b). Therefore, for Mg and Mg alloys in an aqueous solution, the hydrogen evolution phenomenon, which includes cathodic hydrogen evolution (CHE) and ‘anodic hydrogen evolution’ (ACE), is one of the most important features.
Having realized that the hydrogen evolution is closely associated with the corrosion of Mg and its alloys, a simple hydrogen evolution measurement technique was first employed by Song et al. (1997b) to estimate the corrosion rate of Mg. After further illustration of the theory and analysis of possible errors inherent to this method (Song, 2005b, 2006; Song et al., 2001), it has also been widely used on many Mg alloys (Song, 2005a; Song and St John, 2002; Hallope...
Table of contents
- Cover image
- Title page
- Table of Contents
- Copyright
- Contributor contact details
- Preface
- Part I: Surface degradation of light alloys
- Part II: Surface engineering technologies for light alloys
- Part III: Applications for surface engineered light alloys
- Index