
eBook - ePub
Ecotoxicology Essentials
Environmental Contaminants and Their Biological Effects on Animals and Plants
- 500 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Ecotoxicology Essentials
Environmental Contaminants and Their Biological Effects on Animals and Plants
About this book
Ecotoxicology Essentials: Environmental Contaminants and Their Biological Effects on Animals and Plants provides a fundamental understanding of this area for students and professionals in ecotoxicology, ecology, conservation, chemistry, public health, wildlife management, fisheries, and many other disciplines. Although new chemicals and potential problems are developed every year, a basic education is essential to address these new challenges, and this work gives such training. Written with the regulatory framework in mind, the material guides readers on modelling, how to conduct assessments, and human and wildlife risk, focusing on effects on animals rather than transport of chemicals. Simple discussions of chemistry are complemented by coverage on the behavior of the animal, dynamics of the ecosystem, real-life situations like drought, and predators in the system – i.e., the natural system versus the lab setting.
The book's first section contains chapters on the principles of contaminant toxicology including a brief history of the science of ecotoxicology, basic principles of the science, testing methods, and ways of determining if animals have been exposed to either acute or chronic concentrations of contaminants. The second section deals with the primary classes of contaminants including their chemical characteristics, sources, uses, and effects on organisms. The third section focuses on more complex issues such as the regulation of pollution, population and community effects, risk assessment and modelling.
- Uses examples from both aquatic and terrestrial environments and species
- Includes a Terms to Know section and a list of study questions in each chapter, fostering a greater understanding of the issues
- Focuses on the effects of contaminants on wildlife while providing enough chemistry to allow a detailed understanding of the various contaminant groups
- Emphasizes natural examples and 'real' species, rather than laboratory studies on only a handful of organisms
- Features case histories, detailing actual events that include aspects of how the contamination occurred and its effects on wildlife
- Provides material from a wide variety of international sources
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Ecotoxicology Essentials by Donald W. Sparling in PDF and/or ePUB format, as well as other popular books in Biological Sciences & Environmental Science. We have over one million books available in our catalogue for you to explore.
Information
Section II
Chemistry and Effect of Major Families of Contaminants
Outline
Chapter 4
Organochlorine Pesticides
Abstract
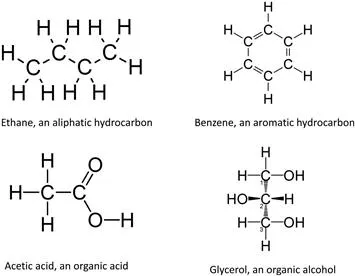
Organic molecules are those that have carbon atoms serving as the backbone or major structural component of the molecule. The simplest group of organic molecules are hydrocarbons that contain only carbon, hydrogen, and oxygen. The carbons in these hydrocarbons can be arranged in straight lines and are called aliphatics (eg, ethane or pentane), or they can be in aromatic ring structures (eg, benzene). The hydrogen and oxygen atoms can also be arranged to form alcohols, organic acids, and other structures. Other elements such as nitrogen, chlorine, bromine, or sulfur can be attached to hydrocarbons to form more complex organic molecules. Organic molecules form the basis for the chemistry of living organisms although they are also found in many nonliving sources, such as petroleum.
Keywords
Aromatic; hydrocarbon; persistent organic pollutant (POP); legacy compounds; dichlorodiphenyltrichloroethane (DDT); biomagnification; stereoisomer
Terms to Know
Aliphatic
Aromatic
Hydrocarbon
Gamma-Aminobutryic Acid (GABA)
Cyclodienes
Persistent Organic Pollutant (POPs)
Legacy Compounds
Stockholm Convention
Stereoisomer
Enantiomer
Diastereomer
Maternal Transfer
Xenobiotic
Apoptosis
Minimal Risk Level (MRL)
Maximum Concentration Level (MCL)
Permissible Exposure Level (PEL)
Introduction
Organic molecules are those that have carbon atoms serving as the backbone or major structural component of the molecule. The simplest group of organic molecules are hydrocarbons that contain only carbon, hydrogen, and oxygen. The carbons in these hydrocarbons can be arranged in straight lines and are called aliphatics (eg, ethane or pentane) (Fig. 4.1), or they can be in aromatic ring structures (eg, benzene). The hydrogen and oxygen atoms can also be arranged to form alcohols, organic acids, and other structures. Other elements such as nitrogen, chlorine, bromine, or sulfur can be attached to hydrocarbons to form more complex organic molecules. Organic molecules form the basis for the chemistry of living organisms although they are also found in many nonliving sources, such as petroleum.
Organochlorine pesticides (OCPs) are molecules that have at least one ring structure and multiple chlorine atoms, thus the name organochlorine. They include many different compounds including dichlorodiphenyltrichloroethane (DDT) and its relatives, gamma-hexachlorocyclohexane (γ-HCH or lindane), methoxychlor, endrin, endosulfan, heptachlor, chlordane, toxaphene, and mirex, to list just a few. Almost all organochlorines have been banned from use in the United States, Canada, and Europe, as well as in some other areas or countries. The intended function of organochlorines was to control insect pests by interfering with their nervous systems. Specifically, DDT and related compounds operate on sodium channels along axons of neurons so that the passage of an “action potential” along the nerve is disrupted. It causes uncontrolled, repetitive, and spontaneous discharges along the axon of a neuron, which most often leads to loss of control in the muscles used for breathing and consequently asphyxiation. A subclass of OCPs called cyclodienes includes endrin, aldrin, endosulfan, etc., and acts on the gamma-aminobutryic acid (GABA) receptors of the neuron. GABA is a very common neurotransmitter in the central nervous system, especially in the cerebral cortex. Cyclodienes bind to the GABA receptors and interfere with the ability of GABA to convey the action potential to the next neuron. Typical symptoms in animals exposed to these pesticides include convulsions, agitation, excitability, and other nervous disorders. In addition to the intended function of OCPs, extensive studies have shown that they have many negative effects including endocrine disruption, cancer, disruption of enzyme functions, and numerous other physiological disorders.
Most OCPs are lipophilic, which means that they readily dissolve in fats and oils, and hydrophobic, which means they do not mix well in water. Due to their lipophilic nature, OCPs bioaccumulate through bioconcentration and biomagnification. Most are very persistent compounds in the environment, remaining for years or decades after application and are part of a larger group of contaminants called persistent organic pollutants (POPs) that also includes dioxins, furans, polychlorinated biphenyls (PCBs), and brominated diphenyl ethers (BDEs) used in fire retardants. Although most OCPs have either been banned or their usage greatly restricted in the United States by the EPA, they are still found in the environment due to their persistent nature and thus, are also called legacy compounds and are used for limited purposes.
Sources and Use
OCPs became popular as insecticides in the 1930s. The most famous, or perhaps infamous, of these pesticides is DDT. The chemical was initially synthesized in 1874 but wasn’t used as a pesticide until 1939. At that time, it was one of the first chemicals to be widely used on farm crops. From then through the 1960s, other OCPs were manufactured in the United States. Based primarily on its risk to human health and persistence, the EPA delisted or banned DDT in 1972, Canada did so a few years later and Mexico banned it in 2000. The US EPA banned other OCPs through 2010; endosulfan is the most recent OCP to be have its registration revoked.
In 1995, the United Nations called for an international examination on the use of POPs. This led to the Stockholm Convention in 2001 which was an international agreement calling for a cessation of 12 of the most noxious pesticides included in five OCP groups: toxaphene, chlordane, and heptachlor; DDT; aldrin, dieldrin, endrin; hexachlorohexane (HCH) and lindane; and pentachlorophenol. The convention also curtailed the production of dioxins and furans. Initially, 128 nations signed the convention and it was instated in 2004. Since then, the total number of signatories is 179. Interestingly, the United States did not internally ratify the convention.
DDT continues to be used for one important human health factor—malaria. There does not seem to be any pesticide currently available that is as cheap and effective in controlling the mosquito vectors of malaria as DDT. Malaria-prone countries in South America, India, and Africa are therefore allowed to use this compound under the UN’s World Health Organization guidelines to treat mosquito netting and spray the walls of their huts or houses, but not to use it in a broad application to wetlands. Unfortunately, the mosquitoes in these countries are developing the same resistance to DDT as was seen in crop pests in the United States prior to its being banned. Additionally, India remains highly conta...
Table of contents
- Cover image
- Title page
- Table of Contents
- Copyright
- Section I: Basic Principles and Tools of Ecotoxicology
- Section II: Chemistry and Effect of Major Families of Contaminants
- Section III: Identifying and Evaluating Large Scale Contaminant Hazards
- Index