
eBook - ePub
Foods, Nutrients and Food Ingredients with Authorised EU Health Claims
Volume 1
- 444 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Foods, Nutrients and Food Ingredients with Authorised EU Health Claims
Volume 1
About this book
Foods, Nutrients and Food Ingredients with Authorised EU Health Claims provides an overview of how health claims are regulated in the European Union, as well as detailed scientific and regulatory information about permitted health claims for particular types of foods and ingredients.
Part one provides a background to the regulation of health claims in Europe. Part two focuses on authorised disease risk reduction claims, claims relating to children's development, and health and proprietary claims. Part three sets out ingredients with permitted "general function claims, including choline, creatine, sweeteners, dietary lactase supplements, and polyphenols in olive oil. Part four outlines foods and nutrients with permitted health claims, with chapters on vitamins and minerals, proteins, meat, fish, water, and the replacement of saturated fats.
Foods, Nutrients and Food Ingredients with Authorised EU Health Claims is the go-to resource for R&D managers and technical managers in the food, and beverage and dietary supplements industry, product development managers, health professionals and academic researchers in the field.
- Provides a comprehensive overview of foods and food substances that have achieved approved health claims in Europe under Regulation EC 1924/2006
- Covers properties and applications of each ingredient, as well as evidence for the health claim and how it benefits consumers
- Outlines the importance of each claim in product development and marketing and regulatory issues such as conditions of use
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Foods, Nutrients and Food Ingredients with Authorised EU Health Claims by Michele Jeanne Sadler in PDF and/or ePUB format, as well as other popular books in Technology & Engineering & Food Science. We have over one million books available in our catalogue for you to explore.
Information
Part I
Regulatory background
Outline
1
The regulation of health claims in Europe*
N. Binns, Independent consultant in nutrition and food regulation, UK
Abstract:
The Regulation on nutrition and health claims made on foods, EC No 1924/ 2006, was published in December 2006, but by December 2013 the implementation of the rules was still incomplete. This chapter provides an overview of the Regulation, describes the development of the lists of claims now in the Community Register, the procedure to approve new claims and summarises the European Food Safety Authority (EFSA) requirements for scientific substantiation.
Key words
EU Regulation; health claims; European Food Safety Authority (EFSA); substantiation
1.1 Introduction
In December 2006 the European Union adopted a Regulation on nutrition and health claims made on foods, EC No 1924/2006 (EU 2006). The Regulation (a corrected version of which was printed in the Official Journal on 18 January 2007) came into force on 19 January 2007 and since then has been gradually implemented – although several transition target dates were not met. An EU Regulation is a piece of legislation that applies automatically across all member states – it is not something that can be implemented into national law with some variation, as can EU Directives. The Regulation consists of a set of recitals that cover the context, scope and intent of the regulation and a set of articles that forms the legally binding part of the text. Although the recitals are not legal text, in terms of interpretation of the legal text or in a case of non-compliance they may be taken into account, as may guidance notes issued by the European Commission (EC) (e.g. EU 2007) or by national competent authorities such as the Department of Health in England (DH 2011).
The main purpose of the Regulation on nutrition and health claims was to provide a high level of protection for the consumer because it was observed that an increasing number of claims were being used on foods. Further, as there were differences in the legal provisions of each member state, the Regulation was intended to harmonise, for the first time across all member states, rules for the use of nutrition and health claims made on foods. In practice, some national differences are likely to persist.
The definition of ‘claim’ is very broad, embracing ‘any message or representation … including pictorial, graphic or symbolic representation in any form which states, suggests or implies that a food has particular characteristics’. Importantly, a brand name or a trade name can also be a claim – although if such a name was in use before 1 January 2005, it does not have to comply with the Regulation until 2022. Nevertheless, there are also certain claims that are out of scope, and, whilst not all are listed as such in the articles of the Regulation, they have been highlighted as out of scope in the recitals or in guidance notes (DH, 2011; EU 2007):
• non-commercial communications, e.g. public health campaigns, scientific articles;
• slogans with no nutrition or health benefit, e.g. ‘have a break, have a Kitkat’;
• terms such as ‘organic’, ‘natural’, ‘traditional’ or ‘fresh’;
• claims such as ‘no additives’ or ‘no added colours’ or ‘free from artificial flavours’;
• allergen and intolerance related claims, e.g. ‘gluten free’ or ‘lactose free’.
The Regulation applies to all commercial communications made about any food (taken to mean food, beverages and food supplements) whether sold in a supermarket or at a canteen or restaurant, as directed to the final consumer. Commercial communications include the label, advertising, leaflets, websites and so on. Although communications directed to health professionals (other than through the scientific literature) are often commercial in nature, they are generally understood not to be within the scope of the regulation. However, the use of ‘health professionals only’ sections of websites, which are accessible to members of the public, has meant that some forms of communication to health professionals may be under scrutiny. The status of communications to the media in the form of press releases and other documents differs per Member State (MS) depending usually on the scope of national, self-regulatory advertising codes.
The Regulation sets out detailed provisions for positive lists of both nutrition claims and health claims; we are only considering health claims in the context of the current reference book. If a claim is not on the relevant list of authorised claims, it cannot be used. Certain types of health claims are prohibited so can never be approved under the current Regulation – these are noted in Table 1.1. Most of these prohibitions are quite straightforward and understandable. Importantly, non-specific claims such as ‘improves health and wellbeing’ or ‘good for you’ or ‘healthy goodness’ may be used only if they are backed up by an authorised health claim that can be made for that food; it is not sufficient to back up a nonspecific claim with an approved nutrition claim. There is, however, some difficulty with Article 12(c) concerning individual health professionals. Further interpretation is needed not only for this prohibition but also relating to the status of national associations of health professions and charities referred to in Article 11.
Table 1.1
Prohibited health claims and related articles
| Prevent, treat, cure human disease | Article 7 Regulation on Food Information (EU, 2011a) |
| Misleading claims | Article 3 NHCR; Article 7 Regulation on Food Information and the Directive on fair trade |
| Non-specific claims (unless accompanied by an approved health claim) | Article 10.3 NHCR |
| Claims that suggest health can be affected by not consuming the food | Article 12 NHCR |
| Rate and amount of weight loss | Article 12 NHCR |
| Claims that make reference to recommendations of individual doctors or health professionals and unapproved associations of health professionals. | Article 12 NHCR |
Abbreviations : NHCR – Regulation 1924/2006 on nutrition and health claims made on foods
Source: EU (2006).
There have been several amendments to the Regulation. The first was to amend the regulatory procedures to cover the new powers of Parliamentary scrutiny granted to the European Parliament (EP) in 2006 but too late to be written into the text of the Regulation (EU 2008a). There was an amendment (EU 2008b) to clarify the definitions under Article 14 and also to allow a more practical transition period for claims directed to children since many claims were already in use in the market. The third amendment was to add several nutrition claims on unsaturated fats to the Annex (EU 2010). A further amending text was published in November 2012 (EU 2012a) allowing the use of ‘no added sodium/salt’ claims and amending the conditions of use for ‘reduced’ claims. Further amendments are inevitable.
1.2 Definitions
A health claim is defined as ‘any claim that states, suggests or implies that a relationship exists between a food category, a food or one of its constituents and health’. However, the subdivision of the claims in the legal text provides for additional levels of complexity (see Fig. 1.1).
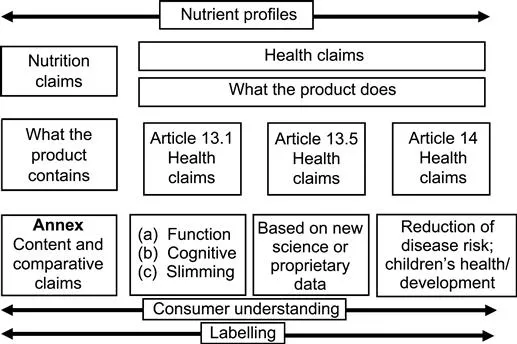
In the legal text health claims are divided into two groups:
• Firstly, the so-called Article 13 claims (as defined in Article 13.1) about the effects of foods or ingredients or nutrients as they relate to growth, development and functions of the body (including weight control and psychological or cognitive effects). For example calcium is good for bones; wheat bran fibre for stool bulking; caffeine keeps you alert; meal replacements help weight management.
• Secondly, Article 14 claims are those referring to a reduction in the risk of a disease risk factor (for example: ‘reduces blood cholesterol; high blood cholesterol is a risk factor for cardiovascular disease’) or claims referring to children’s development and health (e.g. ‘DHA [docosahexaenoic acid] for the development of the eye’).
Article 13 claims are separated in into two further sub-divisions. Article 13.3 claims, which were claims as defined by Article 13.1 that were to be adopted as a Community list of claims under a specific procedure (of which more later). These are variously referred to as ‘Article 13 claims’, ‘Article 13.1 claims’ or, by the European Food Safety Authority (EFSA), as ‘Article 13.2 claims’. The ‘second’ group of Article 13 claims is the so-called Article 13.5 claims. In fact these are exactly the same type of claim as other Article 13 claims from a health/function point of view (as they relate to growth, development and functions of the body) but are differentiated by their being based on ‘new’ science (i.e. the claim is not on the Article 13.3 list) and is thus subject to approval via submission of a dossier and/or are based on data that are proprietary to a company or organisation. Claims authorised on the basis of proprietary data enjoy 5 years exclusivity of use by the owner of the data and their clients after which time the approval must be revalidated (Article 18.5(b)).
The unusual combination of disease risk claims (Article 14.1(a)) and children’s health claims (Article 14.1(b)) was a result of amendments introduced by the European Parliament (EP) during the second reading of the proposed Regulation. These were then adopted during the final negotiations between the European institutions. The purpose of the EP’s amendment was to ensure that claims referring to children’s development and health would require specific approval by the same high level of scientific scrutiny required for disease risk reduction claims rather than being adopted as part of a general list of claims.
In the document published by the EC in December 2007 (EU 2007) there is some guidance about how to determine whether or not a claim is directed at children. One of the issues is that some claims, for example claims around calcium and vitamin D and bone growth and maintenance, are applicable across older age groups as well and are, as such, Article 13 claims. To decide if a claim is an Article 14 claim on children’s development and health you need to consider (a) how the claim is targeted and (b) the nature of the scientific evidence that substantiates the claim. If a claim is directed at children or is substantiated by data that was derived from child studies and is applicable only to them, then the claim is most likely Article 14.1(b). If the data are more general and or the message is relevant to the family, then it may be an Article 13 claim. In other words, some Article 13 claims, for which the scientific substantiation is from studies across the whole population, may be used on products that include children as the target. This is not explicitly written into the guidance, but is the general understanding of what is acce...
Table of contents
- Cover image
- Title page
- Table of Contents
- Copyright
- Contributor contact details
- Woodhead Publishing Series in Food Science, Technology and Nutrition
- Foreword
- Preface
- Part I: Regulatory background
- Part II: Authorised disease risk reduction claims, children’s development and health claims, and proprietary claims
- Part III: Ingredients with permitted ‘general function’ claims
- Part IV: Foods and nutrients with permitted health claims
- Index