
Encapsulation Technologies and Delivery Systems for Food Ingredients and Nutraceuticals
- 640 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Encapsulation Technologies and Delivery Systems for Food Ingredients and Nutraceuticals
About this book
Improved technologies for the encapsulation, protection, release and enhanced bioavailability of food ingredients and nutraceutical components are vital to the development of future foods. Encapsulation technologies and delivery systems for food ingredients and nutraceuticals provides a comprehensive guide to current and emerging techniques.Part one provides an overview of key requirements for food ingredient and nutraceutical delivery systems, discussing challenges in system development and analysis of interaction with the human gastrointestinal tract. Processing technologies for encapsulation and delivery systems are the focus of part two. Spray drying, cooling and chilling are reviewed alongside coextrusion, fluid bed microencapsulation, microencapsulation methods based on biopolymer phase separation, and gelation phenomena in aqueous media. Part three goes on to investigate physicochemical approaches to the production of encapsulation and delivery systems, including the use of micelles and microemulsions, polymeric amphiphiles, liposomes, colloidal emulsions, organogels and hydrogels. Finally, part four reviews characterization and applications of delivery systems, providing industry perspectives on flavour, fish oil, iron micronutrient and probiotic delivery systems.With its distinguished editors and international team of expert contributors, Encapsulation technologies and delivery systems for food ingredients and nutraceuticals is an authoritative guide for both industry and academic researchers interested in encapsulation and controlled release systems.- Provides a comprehensive guide to current and emerging techniques in encapsulation technologies and delivery systems- Chapters in part one provide an overview of key requirements for food ingredient and nutraceutical delivery systems, while part two discusses processing technologies for encapsulation and delivery systems- Later sections investigate physicochemical approaches to the production of encapsulation and delivery systems and review characterization and applications of delivery systems
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
Requirements for food ingredient and nutraceutical delivery systems
Abstract:
1.1 Introduction
1.1.1 Terminology
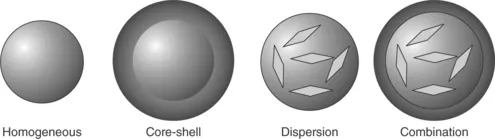




1.1.2 Release mechanisms
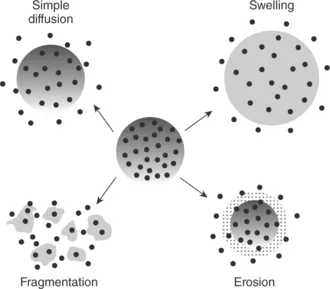
1.2 Active components and the need for encapsulation
Table of contents
- Cover image
- Title page
- Table of Contents
- Copyright
- Contributor contact details
- Woodhead Publishing Series in Food Science, Technology and Nutrition
- Preface
- Part I: Requirements for food ingredient and nutraceutical delivery systems
- Part II: Processing technology approaches to produce encapsulation and delivery systems
- Part III: Physicochemical approaches to produce encapsulation and delivery systems
- Part IV: Characterization and applications of delivery systems
- Index