
- 477 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Practical Protein Crystallography
About this book
Designed for easy use by both beginning and experienced protein crystallographers, the second edition of Practical Protein Crystallography is an essential handbook for any scientist interested in solving a protein structure. The book includes examples of actual experiments and data, electron density maps, and computer methods. This second edition has new material covering CCP4, SHELX, cryocrystallography, MAD and automated fitting.
- In-depth coverage of every aspect of crystallography
- Coverage of the small details that can make or break projects
- Strongly application-oriented
- Exceptionally well illustrated
- Simple and easy-to-follow robust methods
- Tutorials with actual data available on the Web
- Useful for a broad spectrum of scientists
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
1
LABORATORY TECHNIQUES
1.1 PREPARING PROTEIN SAMPLES
A protein sample must be properly prepared before it can be used in a crystal growth experiment. There are many ways that this can be done to accomplish the same goal: to put the protein at a high concentration in a defined buffer solution. Methods of preparing a protein sample that have worked well in our laboratory are outlined here, but if you know of a quicker, easier method then by all means use it.
History and Purification
Since it is not uncommon for one batch of protein to crystallize while the next will not, it is vital to keep a history of each sample and to track each batch separately. Your records may provide the only clue to the differences between samples that produce good crystals and samples that are unusable. For example, there have been several cases where the presence of a trace metal is needed for crystallization. The most famous case is insulin. It seemed that the only insulin that would crystallize was that purified from material collected in a galvanized bucket. It was eventually discovered that zinc was required, and later it was added directly to the crystallization mix. In our lab any sample received is logged into a notebook with a copy of any letters or material sent with the sample.
Samples should be shipped to you on dry ice and kept frozen at –70°C until they are ready for use. Ask the person sending the sample to aliquot the protein into several tubes and to quick-freeze each one. This way you can thaw one aliquot at a time without having to repeatedly freeze–thaw the entire sample, which can damage many proteins. Keep a small portion of the sample apart and save it for future comparison with samples that do not crystallize or crystallize differently.
Always keep protein samples at 4° C in an ice bucket to prevent denaturation and to retard bacterial growth. Perform all sample manipulations at 4°C either in a cold room or on ice. When the samples are finally set up they can be brought to room temperature. Proteins are usually stabilized by the presence of the precipitants used in crystallization, and agents are added to retard microbial and fungal growth. A common antimicrobial agent is 0.02% sodium azide. Other broad-spectrum cocktails sold for use with tissue culture are quite effective.
Exchanging Buffers
If the desired buffer of the sample is not already known, the sample should be placed in a weak buffer near neutrality. A good choice is 50 mM Tris-HCI at pH 7.5 with 0.02% sodium azide. Some proteins will not be stable at low ionic strength, so a small portion should be tried first to see if a precipitate forms. Be sure to wait several hours before deciding if the sample is stable. Observe the sample in a clear glass vial and hold it near a bright light to detect any cloudiness in the sample.
There are two methods for exchanging the buffer solution of the protein sample: dialysis and use of a desalting column. The desalting column is the fastest method, and if disposable desalting columns such as a PD-10 column from Pharmacia-LKB is used, it is very convenient. A single pass through the column will remove 85–90% of the original salt in the sample and, if this is not enough, two passes can be used. Every time the sample is passed over the column it will be diluted about 50%. Unless the sample is very concentrated to begin with, it may be necessary to concentrate the sample after desalting.
Dialysis on small volumes is best carried out in finger-shaped dialysis membrane or with a Microcon (Fig. 1.1). Always soak the membrane first in the buffer to remove the storage solution. A minimum of two changes of dialysis buffer 12 h apart is recommended. The dialysis buffer should be stirred and should be at least 100 times the sample volume. Use membranes with a molecular weight cutoff less than half the sample molecular weight; otherwise you risk losing a substantial portion of the sample. When removing the sample, wash the inside of the membrane with a small amount of buffer to recover the sample completely.
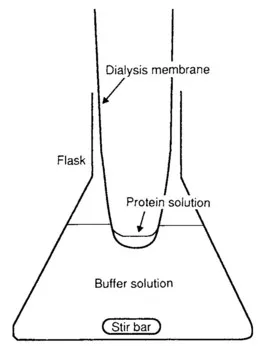
FIG. 1.1 Dialyzing samples to exchange buffers.
Concentrating Samples
First measure the starting concentration of the sample. The simplest way is to measure the absorbance of a 50-times dilution of the sample at 280-nm wavelength and assume the concentration is simply the absorbance times 50. While this method is not very accurate, it is reproducible, and the sample should be pure enough to warrant the assumption that all absorption is due to the protein.1
Always use buffer as a blank and check the buffer versus distilled water to be sure it does not have significant absorption. Some buffers have a significant amount of absorption at 280 nm, which can greatly reduce the accuracy of different absorbance measurements. The diluted absorbance must be below 1.0 or the measurement will be inaccurate. Concentrate the sample to 10–20 mg/ml. If you have enough sample, it is better to concentrate to 30 mg/ml, wash the concentrator with one-half the sample volume, and then add the wash to the sample to make a final concentration of 20 mg/ml. A Centricon is one of the best ways to concentrate the sample. An Amicon will also work well. Another method is to dialyse against polyethylene glycol 20,000 (PEG-20K) using a finger-shaped dialysis membrane. The advantages of this method are that it can be combined with dialysis and that the same membrane used to dialyze the sample can be transferred directly to the PEG-20K for concentrating. The dialysis tube can be put directly onto solid PEG-20K. The water in the sample will be quickly removed, so check the sample often. However, be aware that PEG is often contaminated with salts and/or metals and this may or may not be desirable. In many cases, though, such contamination has actually contributed to crystallization. You may want to keep a sample of the particular batch of PEG you use. If, in the future, a new batch causes problems, you can analyze the differences.
Another method often used is precipitation with ammonium sulfate. If an ammonium sulfate step has already been used in the purification procedure, this may be an easy way to achieve a high concentration. You will want to use a high level of ammonium sulfate to ensure that the entire sample is precipitated. The ammonium sulfate should be added slowly to the solution while kept cold. Let the solution sit for at least 30 min after all ammonium sulfate has dissolved. Spin down the precipitate and remove the supernatant. The pellet can be redissolved in a small amount of buffer. However, since the pellet will contain some salt, a dialysis step will be needed before the protein is ready.
It is not uncommon for a protein to precipitate at high concentrations. If this happens while you are concentrating, add buffer back slowly until all the sample has dissolved. Raising the level of salt by using a more concentrated buffer or by adding sodium chloride can often help stabilize protein solutions. If a precipitate forms, examine it carefully to make sure it is not crystalline. Amorphous precipitates are cloudy and have a matte appearance. Crystalline precipitants are often shiny and if from a colored protein are brightly colored with little cloudy appearance. Two proteins have been crystallized in our lab accidentally during concentration. One was found in an ammonium sulfate precipitation step and the other during concentration on an Amicon to lower the salt concentration.
Storage of Samples
The entire sample will not be used all at once, and the remaining protein solution should be aliquoted and stored frozen at – 70° C. Divide the sample into 100- to 200-μl aliquots in freezer-proof tubes (not glass, which becomes brittle and shatters at low temperatures) and quick-freeze the tubes in an acetone—dry ice or liquid nitrogen bath (Fig. 1.2). Label each tube with the date, a code to identify the sample and the particular batch of the sample, and your initials. Cover the label with transparent tape to prevent the ink from rubbing off when you handle the frozen tubes later. Place the tube in a cardboard box and store in the freezer. It will harm protein samples to be freeze–thawed; although often they may withstand several cycles of freeze–thawing, it is best not to find out the hard way. Thaw the samples in an ice bucket or the cold room when they are to be used. If some sample is left over and it will be used the next day, it can usually be stored at 4° C overnight.
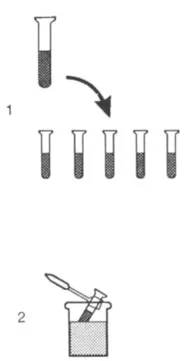
FIG. 1.2 Storage of samples. The procedure involves (1) aliquoting samples into several tubes, then (2) quick-freezing each sample in an acetone—dry ice bath and storing at –70º C.
Ultrapurification
While it is beyond the scope of this handbook to cover purification techniques, the crystallographer has one special technique that is usually not tried by others to further improve the sample: recrystallization (Fig. 1.3). We will assume that you have succeeded in finding conditions that will grow small crystals but are having trouble growing larger ones. It may be worthwhile to recrystallize the sample to improve the purification. A large sample of the protein can be set up in the crystallization mixture and seeded with a crushed crystal. After crystals have grown, you may wish to add slightly more salt to push more protein into the crystalline state. Gently centrifuge down the crystals, or allow them to settle by gravity, and remove the super-natant. Resuspend the crystals in a mother liquor higher in precipitant by about 10% to avoid redissolving and to wash them, and then remove the supernatant. Resuspend the crystals in distilled water to dissolve them. If you have a large amount of precipitate present with the crystals, this method will not remove the precipitate unless it settles the crystals. In these cases, the crystals can be resuspended in 2 ml of artificial mother liquor in a petri dish, then picked up individual crystals with a capillary and manually separated them from the precipitate.
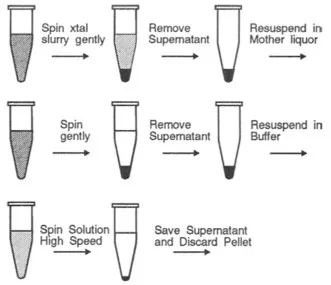
FIG. 1.3 Redissolving crystals.
For the crystals to redissolve well, they should be freshly grown. Old crystals that still diffract well often will not redissolve even in distilled water because the surface of the crystal has become cross-linked. This is especially true of crystals grown from polyethylene glycol.
1.2...
Table of contents
- Cover image
- Title page
- Table of Contents
- Copyright
- FOREWORD TO THE SECOND EDITION
- PREFACE TO THE SECOND EDITION
- ACKNOWLEDGMENTS
- Chapter 1: LABORATORY TECHNIQUES
- Chapter 2: DATA COLLECTION TECHNIQUES
- Chapter 3: COMPUTATIONAL TECHNIQUES
- Chapter 4: XtalView TUTORIALS
- Chapter 5: PROTEIN CRYSTALLOGRAPHY COOKBOOK
- Chapter 6: CRYOCRYSTALLOGRAPHY: BASIC THEORY AND METHODS
- Appendix A: Crystallographic Equations in Computer Code
- Appendix B: Useful Web Sites
- INDEX
- Color Plates
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Practical Protein Crystallography by Duncan E. McRee in PDF and/or ePUB format, as well as other popular books in Biological Sciences & Biochemistry. We have over one million books available in our catalogue for you to explore.