
eBook - ePub
Biological Inorganic Chemistry
A New Introduction to Molecular Structure and Function
- 472 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Biological Inorganic Chemistry
A New Introduction to Molecular Structure and Function
About this book
Biological Inorganic Chemistry: A New Introduction to Molecular Structure and Function, Second Edition, provides a comprehensive discussion of the biochemical aspects of metals in living systems. Beginning with an overview of metals and selected nonmetals in biology, the book then discusses the following concepts: basic coordination chemistry for biologists; structural and molecular biology for chemists; biological ligands for metal ions; intermediary metabolism and bioenergetics; and methods to study metals in biological systems. The book also covers metal assimilation pathways; transport, storage, and homeostasis of metal ions; sodium and potassium channels and pumps; magnesium phosphate metabolism and photoreceptors; calcium and cellular signaling; the catalytic role of several classes of mononuclear zinc enzymes; the biological chemistry of iron; and copper chemistry and biochemistry. In addition, the book discusses nickel and cobalt enzymes; manganese chemistry and biochemistry; molybdenum, tungsten, vanadium, and chromium; non-metals in biology; biomineralization; metals in the brain; metals and neurodegeneration; metals in medicine and metals as drugs; and metals in the environment.
- Winner of a 2013 Textbook Excellence Awards (Texty) from the Text and Academic Authors Association
- Readable style, complemented by anecdotes and footnotes
- Enables the reader to more readily grasp the biological and clinical relevance of the subject
- Color illustrations enable easy visualization of molecular mechanisms
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Biological Inorganic Chemistry by Robert R. Crichton in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Analytic Chemistry. We have over one million books available in our catalogue for you to explore.
Information
Chapter 1
An Overview of Metals and Selected Nonmetals in Biology
Introduction
Why do We Need Anything Other Than C, H, N, and O (together with some P and S)?
What are the Essential Elements and the Essential Metal Ions?
An Idiosyncratic View of the Periodic Table
Introduction
The extraordinarily important role of metals in biology, the environment, and medicine has become increasingly evident over the last twenty to thirty years. Iron- and copper-containing proteins (cytochromes, iron-sulfur proteins, and plastocyanins) are key players in electron transfer, both in the electron-transfer pathways of photosynthetic organisms and in the respiratory chain of mitochondria. Coupling electron transfer with proton pumping across membranes to establish proton gradients is a universal way of generating the currency of cellular free energy, ATP: this constitutes the process which we call oxidative phosphorylation. Photosystem II, which produces oxygen, protons, and electrons from water, which our renewable energy enthusiasts would dearly love to mimic, utilises sophisticated manganese chemistry. Metals like cadmium, manganese, and lead in our environment represent a serious toxic hazard. Even relatively unheard-of elements like polonium can seize the front pages of our national newspapers when their alpha radiation is used to poison a Soviet dissident in London. While many metals are toxic, some metals are used as drugs – cisplatin and related metal-based drugs are used to treat cancer, while lithium, in the form of lithium carbonate, is used in the treatment of manic depression. Modern medicine has increasingly developed noninvasive techniques, both for diagnosis and for therapy. Magnetic resonance imaging depends heavily on the use of paramagnetic metal complexes as contrast agents. A number of metals such as isotopes of cobalt, gallium, and technetium are used as radiopharmaceuticals to deliver sterilizing radiation to targets within the body. A small number of trace elements, like selenium, and the halogens, chlorine and iodine, are also required to ensure human health. While metal deficiencies are well known (for example, inadequate dietary iron causes anemia), it is evident that excessive levels, even of essential metals, can also be toxic – as we will see, this is the case for iron in excess.
It has been clear from the outset that the study of metals in biological systems can only be approached by a multidisciplinary approach, involving many branches of the physical and biological sciences. The study of the roles of metal ions in biological systems represents the exciting and rapidly growing interface between inorganic chemistry and the living world. It has been defined by chemists as bioinorganic chemistry, and by biochemists as inorganic biochemistry. As explained in the Preface, I prefer to use the definition ‘biological inorganic chemistry’ in this book, but would like to indicate to the prospective reader that this text will deal to a much greater extent with the biochemical aspects of metals and other inorganic elements in living systems rather than with their inorganic chemistry.
Why do We Need Anything Other Than C, H, N, and O (together with some P and S)?
The word ‘organic’ itself can have a large number of meanings. The chemical definition is ‘applied to a class of compound substances which naturally exist as constituents of organised bodies (animals or plants), or are formed from compounds which so exist, such as organic acids, bases, molecules, radicals: they all contain or are derived from hydrocarbons’. Hence, organic chemistry is the chemistry of hydrocarbons and their derivatives, or more generally, ‘any chemical compound containing carbon’. However, in this latter definition, some simple compounds of carbon, like carbon dioxide, are sometimes classified as inorganic compounds. Of course, we quickly perceive that carbon alone does not suffice for life – we would not be able to do much with just the three elemental forms of carbon, graphite, diamond, and fullerenes1 (the latter is illustrated below in Fig. 1.1 by the structure of Buckminsterfullerene, a spherical molecule with the formula C60, so named in honor of the geodesic domes of Richard Buckminster Fuller, which they resemble). We also need hydrogen, oxygen, nitrogen, a non-negligible dose of phosphorus, as well as some sulfur.
FIGURE 1.1 Buckminsterfullerene a 60 carbon ‘bucky ball’, made entirely and exclusively of carbon.
It follows that, with the inclusion of oxygen, nitrogen, phosphorus, and sulfur, we escape from the relatively restricted sphere of hydrocarbons made up solely of carbon and hydrogen, and enter a brave new world of organic molecules – acids, aldehydes, ketones, alcohols, amines, sugars, amino acids, and lipids. From these organic building blocks, we can construct proteins, polysaccharides, fats, nucleic acids, even phospholipid bilayers (which together with proteins, constitute the structural leitmotif of biomembranes).
Yet, a living cell does not just require these organic building blocks, together with the biopolymers, and the biomembranes. The enormous negative charges that are generated along the polyphosphate backbone of nucleic acids need to be balanced with appropriate positively charged counter-ions. In order to generate ATP, our universal energy currency, we need to separate proton transport from electron transfer, and use the energy of proton gradients to drive ATP synthesis. While we can transfer electrons using organic molecules like flavins, redox metal ions like iron and copper are much better adapted to this. We need to find ways of amplifying signals, arriving at the cell membrane at nanomolar concentrations, but which result in millimolar intracellular responses. As we move from unicellular organisms to more complex multicellular organisms, we need to generate transmembrane electrical potentials so that we can transmit messages in the form of electrical signals, sometimes over quite long distances. For almost all of these purposes, large, cumbersome and bulky proteins are clearly not the answer. But, perhaps above all else, we must enable the proteins which we call enzymes to catalyse reactions, many of which would quite simply be impossible if we relied solely on organic molecules.
So, if these six elements alone do not enable life as we know it to exist, in its multiple and varied forms – what other elements do we require? Traditionally, whereas organic chemistry concerns compounds of biological origin, inorganic chemistry concerns the properties and behaviour of inorganic compounds, considered to be of mineral origin – inorganic chemistry in French was previously called ‘chimie minérale’(mineral chemistry2). In more recent times, the boundaries between inorganic and organic have become more blurred – many inorganic compounds contain organic ligands, while, as mentioned earlier, some carbon-containing compounds are traditionally considered inorganic, and many organic compounds contain metals. As we will see in the next section, in the course of evolution, Nature has selected constituents not only from the organic world, but also from the inorganic world to construct living organisms. Many of these are metals, elements to the left of the periodic table, which readily lose their valence electrons to form cations.
There is an interesting historical illustration of this requirement for metals in catalysis. The celebrated German chemist Richard Willstätter (Chemistry Nobel Prize, 1915) proposed that enzymes were not proteins – in his view, the protein was only a carrier for the veritable catalytic centre (he called the protein “nur ein träger Substanz”). In 1929, James Sumner accidentally left a preparation of urease from jack bean (the enzyme which catalyses the decomposition of urea to ammonia and carbon dioxide) on a laboratory table overnight. The night was cold, and to his surprise, the following morning, he found that the protein had crystallised. Together with John Northrop, who crystallised pepsin and trypsin, the conclusive proof of the protein nature of enzymes was thereby established (they both received the Chemistry Nobel Prize in 1946). Although their discovery appeared to have disproved Willstätter’s theory, he was vindicated some 50 years later by the demonstration that urease is in fact a nickel-dependent enzyme, and that when the Ni is removed, urease loses its catalytic activity. Of course, with the benefit of hindsight, we can see that both viewpoints were correct. The protein is indeed a carrier for the Ni, but a carrier which provides the right coordination sphere3 to bind the Ni in the right conformation, as well as creating the right environment for the molecular recognition of the substrates, urea and water, and their binding in the right orientation to enable the di-metallic nickel site to carry out its catalysis (see Chapter 15 for more details).
What are the Essential Elements and the Essential Metal Ions?
Just six elements – oxygen, carbon, hydrogen, nitrogen, calcium, and phosphorus – make up almost 98.5% of the elemental composition of the human body by weight. Just 11 elements account for 99.9% of the human body (the additional five are potassium, sulfur, sodium, magnesium, and chlorine). However, as we will see shortly, we can identify between 22 and 30 elements which are required by some, if not all, living organisms. Many of these are metals: some of them, like sodium, potassium, calcium, and magnesium, are present in quite large concentrations, and are known as ‘bulk elements’. Indeed, these four cations constitute nearly 99% of the metal ion content of the human body. Others, like cobalt, copper, iron, manganese, molybdenum, and zinc, are known as ‘trace elements’, with dietary requirements that are much lower than the bulk elements; yet, they are no less indispensable for human life.
We now discuss just why these elements out of the entire periodic table have been selected. One thing is clear – they were not only selected as a function of their abundance and their availability in the universe as well as in the earth’s crust, and the oceans (which constitute the major proportion of the earth’s surface), but also on the basis of their suitability for the functions that they are called upon to play, in what is predominantly an aqueous environment.4
It therefore comes as no great surprise that within our solar system itself, all 11 of the principal elements found in man are in the top 20 in terms of abundance, with five of them figuring in the top ten – hydrogen, carbon, nitrogen, oxygen, and sulfur. When we consider the abundance of these 11 obviously essential elements in the earth’s crust (Fig. 1.2), we find that no less than six of them (hydrogen, oxygen, and the four alkali and alkaline earth metals cited above – sodium and potassium, magnesium, and calcium) are among the top ten (together with aluminium, silicon, titanium and, not surprisingly, iron, since the earth’s core is predominantly constituted by iron, together with significant amounts of nickel). The remaining five are among the top 20.
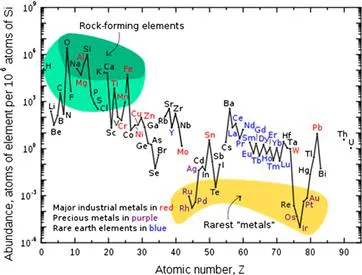
FIGURE 1.2 Abundance (atom fraction) of the chemical elements in Earth’s upper continental crust as a function of atomic number.
But we have every reason to believe that life, as we know it, originated from the oceans, so we also need to consider the distribution of the eleven essential elements in this environment. This is, of course, influenced by the solubility of the corresponding element in salt water. So, it is no surprise that today we find very low concentrations of iron in the oceans (although, if the primitive atmosphere was, as we think, reducing, divalent ferrous iron would have been readily available in a soluble form). So, of our eleven key elements, how many are now found in the water of our oceans? Clearly, sodium and chlorine for starters, but hydrogen, oxygen, and carbon, together with magnesium, sulfur, calcium, potassium, and bromine, make the top 10. The only two which do not mak...
Table of contents
- Cover Image
- Table of Contents
- Title
- Copyright
- Preface to the 2nd Edition
- Chapter 1. An Overview of Metals and Selected Nonmetals in Biology
- Chapter 2. Basic Coordination Chemistry for Biologists
- Chapter 3. Structural and Molecular Biology for Chemists
- Chapter 4. Biological Ligands for Metal Ions
- Chapter 5. An Overview of Intermediary Metabolism and Bioenergetics
- Chapter 6. Methods to Study Metals in Biological Systems
- Chapter 7. Metal Assimilation Pathways
- Chapter 8. Transport, Storage, and Homeostasis of Metal Ions
- Chapter 9. Sodium and Potassium – Channels and Pumps
- Chapter 10. Magnesium–Phosphate Metabolism and Photoreceptors
- Chapter 11. Calcium – Cellular Signalling
- Chapter 12. Zinc – Lewis Acid and Gene Regulator
- Chapter 13. Iron
- Chapter 14. Copper – Coping with Dioxygen
- Chapter 15. Nickel and Cobalt
- Chapter 16. Manganese – Oxygen Generation and Detoxification
- Chapter 17. Molybdenum, Tungsten, Vanadium, and Chromium
- Chapter 18. Non-metals in Biology
- Chapter 19. Biomineralisation
- Chapter 20. Metals in Brain
- Chapter 21. Metals and Neurodegeneration
- Chapter 22. Metals in Medicine and Metals as Drugs
- Chapter 23. Metals in the Environment
- Index