
- 568 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Principles of Asymmetric Synthesis
About this book
The world is chiral. Most of the molecules in it are chiral, and asymmetric synthesis is an important means by which enantiopure chiral molecules may be obtained for study and sale. Using examples from the literature of asymmetric synthesis, this book presents a detailed analysis of the factors that govern stereoselectivity in organic reactions.
After an explanation of the basic physical-organic principles governing stereoselective reactions, the authors provide a detailed, annotated glossary of stereochemical terms. A chapter on "Practical Aspects of Asymmetric Synthesis" provides a critical overview of the most common methods for the preparation of enantiomerically pure compounds, techniques for analysis of stereoisomers using chromatographic, spectroscopic, and chiroptical methods.
The authors then present an overview of the most important methods in contemporary asymmetric synthesis organized by reaction type. Thus, there are four chapters on carbon-carbon bond forming reactions, one chapter on reductions, and one on oxidations (carbon-oxygen and carbon-nitrogen bond forming reactions). This organization allows the reader to compare the leading methods for asymmetric synthesis in an appropriate context.
A highlight of the book is the presentation and discussion of transition states at the current level of understanding, for important reaction types. In addition, extensive tables of examples are used to give the reader an appreciation for the scope of each reaction. Finally, leading references are provided to natural product synthesis that has been accomplished using a given reaction as a key step.
- Authoritative glossary to aid understanding of stereochemical terminology
- Explanations of the key factors influencing stereoselectivity with numerous examples, organized by reaction type
- A handy reference guide to the literature of asymmetric synthesis for practitioners in the field
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
Chapter 1
Introduction, General Principles, and Glossary of Stereochemical Terms
1.1 Why We Do Asymmetric Syntheses
L’univers est dissymétrique
Louis Pasteur (1874)
In modern terminology, Pasteur would say “The universe is chiral.”1 We are constantly learning more about the implications of chirality, from weak bosons in nuclear physics to the origins of life on earth and the double helix of DNA [1–5]. Most organic compounds are chiral. Chemists working with perfumes, cosmetics, nutrients, flavors, pesticides, vitamins, and pharmaceuticals, to name a few examples [6–11], require access to enantiomerically pure compounds. Single enantiomer formulations now account for most of the chiral drugs on the market. One estimate suggests that approximately half of the worldwide revenues from chiral products were the result of traditional synthesis from the chiral pool or resolution, whereas less than half result from chemical catalysis [12].
As our ability to produce enantiomerically pure compounds grows, so does our awareness of the differences in pharmacological properties that a chiral compound may have when compared with its enantiomer or racemate [13–19]. We easily recognize that all biological receptors are chiral, and as such can distinguish between the two enantiomers of a ligand or a substrate. Enantiomeric compounds often have different odors or tastes [20–22].2 Thus, it is obvious that two enantiomers should be considered different compounds when screened for pharmacological activity [10,13,23]. The demand for enantiomerically pure compounds as drug candidates is not likely to let up in the foreseeable future.
How might we obtain enantiomerically pure compounds? Historically, the best answer to that question has been to isolate them from natural sources. Derivatization of natural products or their use as synthetic starting materials has long been a useful tool in the hands of the synthetic chemist, but it has now been raised to an art form by some practitioners, wherein complex molecules are dissected into chiral fragments that may be obtained from natural products [24–34]. Even today, there is no way of obtaining enantiomerically pure compounds without ultimately resorting to Nature, whether for a building block, an auxiliary, or a catalyst.
So if the objective is to obtain an enantiomerically pure compound, one has a choice to make: synthesize the molecule in racemic form and resolve it [35], find a plant or a bacterium that will make it for you, start with a natural product such as a carbohydrate, terpene, or alkaloid (but beware of racemic or partly racemic natural products), or plan an asymmetric synthesis. Among the factors to consider in weighing the alternatives are the amount of material required, the cost of the starting materials, length of synthetic plan, etc., factors that have long been important to synthetic design [36–41]. For the purposes of biological evaluation, it may be desirable to include a resolution so that one synthesis will provide both enantiomers. But for the production of a single enantiomer, a classical resolution will have a maximum theoretical yield of 50% unless the unwanted enantiomer can be recovered and recycled, or the process is stereoconvergent via an asymmetric transformation or a dynamic resolution. In most cases, starting with a natural product will be restricted to the production of only one enantiomer by a given route, notwithstanding the talent of some investigators to produce both enantiomers of a target from the same chiral starting material. Such practical aspects are discussed more fully in Chapter 2.
1.2 What is an Asymmetric Synthesis?
The most quoted definition of an asymmetric synthesis is that published by Marckwald in 1904 [42]:
“Asymmetrische” Synthesen sind solche, welche aus symmetrisch constituirten Verbindungen unter intermediärer Benutzung optisch-activer Stoffe, aber unter Vermeidung jedes analytischen Vorganges, optisch-activ Substanzen erzeugen.3
In modern terminology, the core of Marckwald’s definition is the conversion of an achiral substance into a chiral, nonracemic one by the action of a chiral reagent. Marckwald’s point of reference, of course, was biochemical processes, so it follows that enzymatic processes [43–45] are included by this definition. Marckwald also asserted that the nature of the reaction was irrelevant, so a self-immolative reaction or sequence4 such as an intermolecular chirality transfer in a Meerwein–Pondorf–Verley reaction would also be included:

Interestingly, the Marckwald definition is taken from a paper that was rebutting a criticism [46] of Marckwald’s claim to have achieved an asymmetric synthesis by a group-selective decarboxylation of the brucine salt of 2-ethyl-2-methylmalonic acid [47,48]:
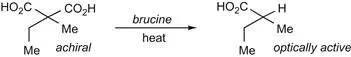
Thus, from the very beginning, the definition of what an asymmetric synthesis might encompass, or even if one was possible, has been a matter of discussion. On the l...
Table of contents
- Cover image
- Title page
- Table of Contents
- Copyright
- Dedication
- Foreword
- Preface
- Chapter 1. Introduction, General Principles, and Glossary of Stereochemical Terms
- Chapter 2. Practical Aspects of Asymmetric Synthesis
- Chapter 3. Enolate, Azaenolate, and Organolithium Alkylations
- Chapter 4. 1,2- and 1,4-Additions to C=X Bonds
- Chapter 5. Aldol and Michael Additions of Allyls, Enolates, and Enolate Equivalents
- Chapter 6. Cycloadditions and Rearrangements
- Chapter 7. Reductions and Hydroborations
- Chapter 8. Oxidations
- Index
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Principles of Asymmetric Synthesis by Robert E. Gawley,Jeffrey Aube in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Organic Chemistry. We have over one million books available in our catalogue for you to explore.