
Understanding the Science of Food
From molecules to mouthfeel
- 424 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
About this book
Being able to understand the principles of food science is vital for the study of food, nutrition and the culinary arts. In this innovative text, the authors explain in straightforward and accessible terms the theory and application of chemistry to these fields.
The key processes in food preparation and the chemistry behind them are described in detail, including denaturation and coagulation of proteins, gelatinisation, gelation and retrogradation of starches, thickening and gelling, browning reactions, emulsification, foams and spherification, chemical, mechanical and biological leaveners and fermentation and preservation.
The text also describes the science of key cooking techniques, the science of the senses and the experience of food, food regulations and the future of healthy food. The origins of food are explored through a focus on the primary production of key staples and their journey to the table. Tips and advice from leading chefs as well as insights into emerging food science and cutting-edge nutrition research from around the world are included throughout, and reveal both the practical application of food chemistry and the importance of this field.
Featuring explanatory diagrams and illustrations throughout, Understanding the Science of Food is destined to become an essential reference for both students and professionals.
'An innovative and informative text that will address the need for a food science text suitable for nutrition and dietetics students in Australia.' - Katherine Hanna, Faculty of Health, Queensland University of Technology.
'A unique and timely text that will be welcomed by students, instructors, and scientists in multiple disciplines. I am thrilled to see such a modern take on the subject, blending the fundamentals of food science and chemistry with the insights and experience of practitioners from the culinary arts.' - Patrick Spicer, lecturer and researcher in food science
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
Part 1
FOOD COMPONENTS,
INTRODUCTORY CHEMISTRY
AND FOOD SAFETY
Chapter 1
Introductory chemistry
- describe atoms, elements, molecules, compounds and ions
- explain chemical bonding, including the different bonding types and their relative strengths
- outline the periodic table, including how periods, groups and blocks of elements are arranged and the reasons for these arrangements
- identify, name and draw key organic compounds found in food in a range of formats
- explain oxidation and reduction reactions
- explain acid–base reactions and how buffers work.
Foundations
Atoms, elements, molecules and compounds
Atoms
| Shell | Subshell | |||
| Number | Name | Max. electrons | Name | Max. electrons |
| 1 | K | 2 | 1s | 2 |
| 2 | L | 8 | 2s | 2 |
| 2P | 6 | |||
| 3 | M | 18 | 3s | 2 |
| 3p | 6 | |||
| 3d | 10 | |||
| 4 | N | 32 | 4s | 2 |
| 4p | 6 | |||
| 4d | 10 | |||
| 4f | 14 | |||
| 5 | O | 50 | 5s | 2 |
| 5p | 6 | |||
| 5d | 10 | |||
| 5f | 14 | |||
| 5g | 18 | |||
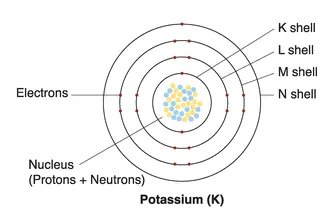
Note: This figure is not to scale, and the electrons are in constant motion.
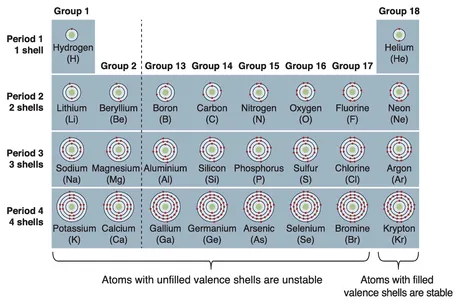
Elements
Table of contents
- Cover
- Half Title
- Title Page
- Copyright Page
- Contents
- List of figures, tables and boxes
- Units of measurement
- About the authors
- Introduction
- Part 1 Food components, introductory chemistry and food safety
- Part 2 Unlocking key food chemistry reactions
- Part 3 Enhancing cooking
- Appendix 1 Food regulations in Australia and New Zealand
- Appendix 2 A scientific approach in the kitchen
- Index