
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Applied Colloid and Surface Chemistry
About this book
An updated guide to the interaction between solids, liquids, and gases and their application to numerous everyday processes
The revised and updated second edition of Applied Colloid and Surface Chemistry offers a comprehensive introduction to this interdisciplinary field that takes a practical approach and includes information on applications drawn from a wide range of industries. The easy-to-follow text contains new content that focuses on applications such as the prevention of propeller cavitation, industrial explosives, PFAS contamination, and bubble column evaporators.
With contributions from noted experts on the topic, the book contains keynote sections written by practicing industrial research scientists, who highlight real-world industrial examples. These examples range from water treatment through to soil management as well as examples from the coatings and photographic industries. Designed as an accessible resource, the book separates the more demanding mathematical derivations from the main text. The text features approachable, structured chapters, learning objectives, tutorial questions with answers, and explanatory notes. This important book:
- Offers a combination of physicochemical background, industrial, and everyday applications and experiments
- Underlines the importance of colloidal sciences in science and industry
- Presents real-world industrial applications
- Includes tried and tested laboratory experiments
Written for students of chemistry, materials science, and engineering, Applied Colloid and Surface Chemistry, Second Edition offers an updated guide to soft matter presenting the bridge between science, with proven laboratory experiments, and real-world industrial applications.
Information
1
Introduction
INTRODUCTION TO THE NATURE OF COLLOIDAL SOLUTIONS
Historical note: The term ‘colloid’ is derived from the Greek word for glue, ‘kolla’. It was originally used for gelatinous polymer colloids, which were identified by Thomas Graham in 1860 in experiments on osmosis and diffusion.
- A gravitational force, tending to settle or raise particles depending on their density relative to the solvent.
- A viscous drag force, which arises as a resistance to motion, since the fluid has to be forced apart as the particle moves through it.
- The ‘natural’ kinetic energy of particles and molecules, which causes random Brownian motion.
| r/Å | 100 | 1000 | 10,000 | 105 | 106 |
| r/μm | 0.01 | 0.1 | 1 | 10 | 100 |
| V/cm/sec | 2 × 10−8 | 2 × 10−6 | 2 ×10‐4 | 2 × 10−2 | 2 |
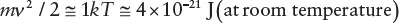
| r/Å | 100 | 1000 | 10,000 | 105 | 106 |
| r/μm | 0.01 | 0.1 | 1 | 10 | 100 |
| ν/cm/sec | 102 | 3 | 0.1 | 3 × 10−3 | 1 × 10‐4 |
Table of contents
- Cover
- Table of Contents
- Title Page
- Copyright Page
- Dedication Page
- Preface
- About the Companion Website
- 1 Introduction
- 2 Surface Tension and Wetting
- 3 The Prevention of Fluid Cavitation
- 4 Thermodynamics of Adsorption
- 5 Surfactants and Self‐Assembly
- 6 PFAS Contamination
- 7 Emulsions and Microemulsions
- 8 Charged Colloids
- 9 Van Der Waals Forces and Colloid Stability
- 10 Bubble Coalescence, Foams and Thin Surfactant Films
- 11 Bubble Column Evaporators
- Appendices
- Index
- End User License Agreement
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app