Canine and Feline Cytology - E-Book
Canine and Feline Cytology - E-Book
- 544 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
About this book
"This is a 'go-to' reference text for a serious cytologist. " Reviewed by: Kathleen Tennant on behalf of Veterinary Record, November 2015- Comprehensive coverage of all body systems and body fluids — and the pathological changes associated with various infectious agents — emphasizes areas in which the application of cytology has the greatest diagnostic value.- Exceptional-quality, full-color photomicrographs show both normal and abnormal tissue and also include detailed legends.- Discussions of clinical, differential, and cytological diagnosis accompany the illustrations of lesions and conditions in each chapter.- Helpful hints for improving specimen quality are provided in discussions of common errors and problems encountered in the preparation of cytological specimens.- Coverage of histology in organ system chapters demonstrates the histological or histopathologic corollary of cytologic findings.- Clear, concise descriptions include sampling techniques, slide preparation and examination, and guidelines for interpretation, leading to accurate in-house and commercial laboratory diagnosis.- Easy-to-use, well-organized format includes many tables, algorithms, boxes, and Key Point callouts for at-a-glance reference.- NEW! Chapter on Fecal Cytology- Highlighted boxes featuring Key Points provide helpful tips for best conceptual understanding and diagnostic effectiveness- Photomicrographs now include more comparative histology- Discussions of broader uses of stains and immunocytochemistry for differential cytologic characterization- Expanded chapter on Advanced Diagnostic Techniques includes more methodology and application of current tools, representing advances in both aspiration and exfoliative cytology.
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
The Acquisition and Management of Cytology Specimens
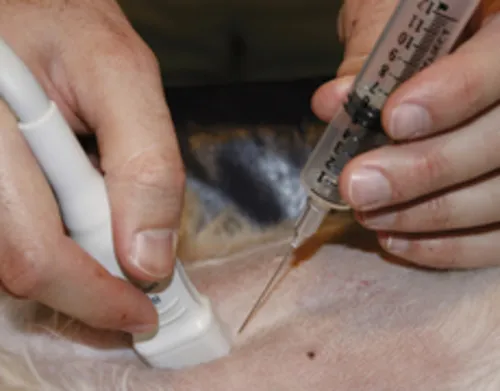
General Sampling Guidelines
| Biopsy Technique | Specimen | Preparation Technique |
A. Aspiration of Solid Tissue 1. Suction 2. Nonsuction | ||
| unknown mass | squash, suspension cytospin | |
| vascular tissue | squash, blood smear | |
B. Fluid Aspiration 1. Bloody fluid 2. Non-bloody fluid 3. EDTA syringe | ||
| effusion (pericardial) | buffy coat smear | |
| effusions, synovial fluid, cerebrospinal fluid, urine | direct, sediment, cytospin | |
| bone marrow | particle squash | |
C. Incisional Biopsy | soft tissue, bone marrow core | imprint, tissue roll |
D. Excisional Biopsy | masses, lymph node, eye, testicle | imprint |
E. Scraping | firm tissue, conjunctiva | imprint, spread, squash |
F. Swab | vaginal, fecal, oral, ocular | imprint, roll |
G. Washes | prostate, urinary bladder, respiratory, peritoneal lavage | sediment, cytospin |
Diagnostic Imaging-Guided Sample Collection
Biopsy Guidance
Table of contents
- Cover image
- Title page
- Table of Contents
- Copyright
- Contributors
- Dedication
- Preface
- Acknowledgments
- Chapter 1. The Acquisition and Management of Cytology Specimens
- Chapter 2. General Categories of Cytologic Interpretation
- Chapter 3. Skin and Subcutaneous Tissues
- Chapter 4. Hemolymphatic System
- Chapter 5. Respiratory Tract
- Chapter 6. Body Cavity Fluids
- Chapter 7. Oral Cavity, Gastrointestinal Tract, and Associated Structures
- Chapter 8. Dry-Mount Fecal Cytology
- Chapter 9. The Liver
- Chapter 10. Urinary Tract
- Chapter 11. Microscopic Examination of the Urinary Sediment
- Chapter 12. Reproductive System
- Chapter 13. Musculoskeletal System
- Chapter 14. The Central Nervous System
- Chapter 15. Eyes and Adnexa
- Chapter 16. Endocrine/Neuroendocrine System
- Chapter 17. Advanced Diagnostic Techniques
- Appendix 1. Microscope Basics and Telecytology
- Appendix 2. Selected Cytologic Stains and Techniques
- Appendix 3. Interference and Polarizing Substances
- Appendix 4. Chromatin Patterns
- Appendix 5. Advanced Collection and Preparation Techniques
- Appendix 6. Immunocytochemistry Staining Protocol
- Appendix 7. List of Specialized Diagnostic Testing Sites
- Appendix 8. Quality Assurance and Diagnostic Test Reporting
- Index