![]()
Chapter One
Fire and Earth
CREATING COMBUSTION
According to many myths, we became truly human only when we acquired fire. So it is natural to assume a parallel awakening for the place we live. Rather, the Earth likely simmered through more than four billion years before its biotic broth boiled over. Some of fire's components the ancient Earth acquired only after long eons. Even more critically, those ingredients needed a durable context in which to mingle. The parts had to combine and do so consistently. Combustion has its creation story. Fire has its history.
Of fire's three essential elements, only the heat of ignition thrived on the early Earth. Oxygen did not begin to collect until the last two billion years, and did not begin to approach modern quantities until roughly 500 million years ago. Land plants suitable to carry combustion did not become abundant until 400 million years ago. Before that time the Earth lacked the means to burn regularly or vigorously. It is possible that aquatic biomass might have burned, if a lagoon or marsh dried or storms hurled kelp or algal mats into deep berms where they dried, met lightning or lava, and combusted. But such burns, if they occurred, would little resemble modern fires, and are ecological freaks, never absorbed or ordered within a biological community. Earth's original fires—its colonizing fires—demanded land plants. Probably these consisted of primordial moors, a matrix of near-shore organic peat and reeds. Fires probably first flickered during the early Devonian, roughly 400 million years ago. The most ancient fossil charcoal dates from that epoch.
Since then, fire's evolution has been unending if uneven. Each of combustion's components has existed more or less distinctly from the others, colliding from time to time with a fizz of oxidation or a brilliant burst of burning. But combustion could survive only if it had a consistent and durable context. Over time, fire became itself a synthesizing process, a kind of biochemical flywheel that has helped to balance its separate parts into a coherent whole. It has affected the chemistry of the atmosphere. It has influenced, perhaps profoundly, the character of life. Progressively, the biosphere has absorbed fire and tweaked it to fit a system of biological checks and balances. This was easiest with oxygen and fuel, both the products of life. The absorption of ignition proved more vexing, and had to await the arrival of creatures who could make sparks and heat as easily as they could drill bone and chip flint. Those creatures, of course, were ourselves.
How Fire Came to Be
Casting Sparks
Combustion requires a spark. It needs a jolt of energy to unpack photosynthesized matter, to set off a chain reaction that can release enough surplus energy through oxidation to continue. The early Earth offered several sources: falling rocks, volcanic discharges, extraterrestrial impacts, and lightning. After dead biomass collected in heaps, spontaneous combustion was selectively possible, and after fossil fuels were exposed, coalfields, petroleum seeps, and oil shales could take fire and hold it for centuries, even millennia. But of this geophysical exotica, only lightning is sufficiently consistent and universal to account for the natural history of fire.
Volcanoes are a faux fire, but they have the capacity to kindle real ones. Flowing lava instantly burns what it touches; eruptions often spawn thunderstorms, which hurl down lightning like volcanic bombs. But while widespread across geologic time, volcanoes are fixed in geographic space. Moreover, volcanic-kindled fires burn locally; lava or ash covers the burns, as often as not; and one way or another the overall disturbance of the volcano smothers the effects of the fire. In brief, volcanoes are too few, too small, too rare to account for the near-universal realm of fire. Most of the burning Earth is far removed from spark-casting volcanoes.
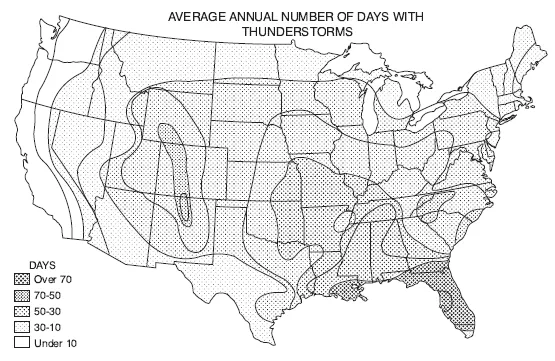
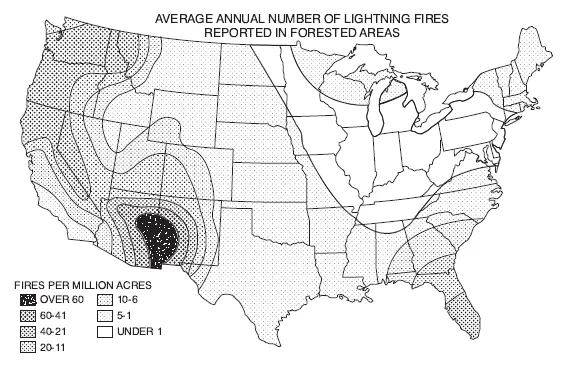
FIGURE 1. The wet and the dry. Patterns of wetting and drying shape ignition as well as fuels. Areas without lightning lack natural fires because there is no spark; but spark alone is not sufficient if heavy rain accompanies it. The geography of lightning is not identical to the geography of lightning-caused fire.
Consider the United States. A map of thunderstorm days (top) shows a concentration of lightning in the Southeast. A map of lightning-kindled forest fires (bottom), however, highlights the West. In particular, the Southwest boasts an ideal formula for fire. A long droughty spring ends in a “monsoon” announced by sporadic summer thunderstorms, beginning in early July. In their first surge, the storms are scattered, some towering over superheated deserts or more commonly over isolated mountains and high rims. Often these thunderstorms are so dry that the rain evaporates before it reaches the ground or soon afterward. There is enough moisture to power a storm but not enough to saturate the surface fuels. As the rainy season progresses, more fires start but fewer become large as the grasses green up and the woody stems flush. (Sources: Schroeder and Buck 1970, and Yearbook of Agriculture 1941, both redrawn by the University of Wisconsin Cartographic Lab)
That leaves lightning. Not every place experiences lightning, and not every lightning-blasted place knows fire. The natural history of lightning fire is lumpy: the fires come in bursts, they crowd in time, they bunch in space. Some years have many, some have few. Although some places never know them, some feel them annually, or until climate reshuffles the deck of places wetted and dried. But its longevity, geography, and concentrated heat mean that lightning clearly accounts for the fact that fire is geologically old and geographically extensive.
Even so, only a tiny fraction of lightning kindles fire. Only one bolt in four reaches the ground. Most of those strike rock or sea, or slam into fuels too wet or shattered or misarranged to burn. Of those that hit something combustible, only one in five has the right properties to convert electrical charge into combustion, the “hot” lightning with high amperage and low voltage. (High-voltage “cold” lightning tends to blast without burning.) Moreover, the storm that looses lightning also dumps rain. What the first can start, the second can stop. The geography of lightning thus overlaps only lightly with the geography of fire. Rather, fire burns along the margins—with the first storms after a long drought, or from dry storms whose veils of rain evaporate before reaching the ground, or in regions prone to severe swings of wet and dry conditions. However often lightning rolls the dice, the house odds remain against fire.
Yet ignition occurs often enough to render lightning fire the vestal flame of the ancient Earth. In some landscapes it is fickle if powerful, rather like hurricanes. In America, for example, dry thunderstorms can charge whole regions with fire. Between 1946 and 1973, “fire busts” in the Northern Rockies splattered the national forests with more than 100 fires a day on 25 occasions; five times, the total exceeded 200 fires. On July 17, 1940, there were 335 fires. Over a ten-day period this same storm kindled 1,488 fires. Between 1960 and 1974, in the national forests of the Southwest five times lightning kindled in excess of 500 fires over a ten-day period. The region averages roughly 2,000 such fires a year. Here lightning fire is as much a chronic presence as storm and sunlight. But wherever it occurs the biota adjusts. Some trees are struck more often than others; curiously, these tend to be species best adapted to survive fire. The lightning bolts that relentlessly restore electrical equilibrium to the Earth also maintain its biological equilibrium.
Yet they do so in peculiar ways. While trees may adapt to lightning, lightning does not adapt to trees. It knows no biological feedback. If lightning has, over geologic time, been the most persistent of fire's elements, it is also the most inflexible. It obeys a geophysical logic, a cold spark without biological control. It matters not to lightning whether it strikes granite or lodgepole pine, a lake or a barn, a sodden log or a snag as parched as kiln-dried lumber. Lightning rips through Jupiter's atmosphere as much as Earth's.
Oxygen and biomass could not ignore the biosphere: life created them. They would have to interact, and combustion would coevolve along with them. But fire's primordial instrument of ignition could exist on its own, leaving fire without a biological means for regulating spark as it did fuel and oxygen. Or rather, it lacked such means until hominids wrested ignition away from lightning's virtual monopoly. From that moment on, the most rigid element of fire's combustion triangle became the most pliant; and a process that had depended on an electrical charge—its bolts as precise as a rifle shot and as random as storm winds—became a global spark as common as grass and as universal as humanity's restless hand and roving mind.
Making Air
Lightning can spark a reaction, but it cannot sustain one. For the act of kindling to yield to self-sustaining fire, free oxygen has to flow into the combustion zone. Yet only in the last two billion years has the Earth succeeded in filling its atmosphere with oxygen on any scale. For several hundred million years thereafter, the atmosphere's oxygen content waxed and waned. During the Carboniferous and Permian, it swelled to perhaps 35 percent, which made possible a general giantism—beetles the size of puppies and dragonflies as big as ravens. By 150 million years ago it stabilized at 21 percent. For this immense shift, without which fire could not exist, the evolution of life is responsible. Plants pumped out more oxygen than the early Earth could absorb.
That early Earth produced some oxygen by splitting volcanically outgassed molecules of water and carbon dioxide. Such photolysis, along with the chemical weathering of metallic oxides, spewed out packets of free oxygen. But the early Earth's atmosphere was a reducing one. Whatever oxygen that photolysis could produce was quickly absorbed as the freed molecules bonded to carbon, hydrogen, iron, and sulfur—all of them ravenous scavengers of free oxygen. The earliest life (perhaps around 3.5 billion years ago) emerged without oxygen. The first photosynthesizers (roughly 3.1 billion years ago) were also anaerobic. The chemical avidity of free oxygen probably threatened them and likely proved toxic.
The shift from an atmosphere empty of oxygen to one rich in it occurred when oxygen sources increased and oxygen sinks diminished. A critical moment came about 2.3 billion years ago when the earliest photosynthesizing prokaryotes appeared in the form of seaborne blue-green algae. They bolstered oxygen's sources on a large scale by releasing it as a byproduct; yet what algae pumped out, rocks soaked up. Vast quantities of iron, sulfur, and especially carbon were oxidizing and settling into the sedimentary lakes of geologic time. By 1.8 billion years ago, oxidized iron had become abundant in the geologic record, followed by carbonates. In the beginning, carbon dioxide had dominated the Earth's atmosphere; but after eons of lithic burial in forms like limestone, it was becoming a mere trace element, replaced in bulk by the more inert nitrogen. Eventually oxygen's sinks began reaching full capacity and free oxygen flooded the atmosphere. The living world, like the geologic, had to accommodate it. Gradually, organisms transformed a threat into an opportunity. Around 1.3 billion years ago, aerobic photosynthesis emerged, further soaking the Earth's air. About 600 million years ago, select organisms learned to exploit the oxygen that surrounded them to split apart what photosynthesis had joined. Aerobic respiration became common, and a chemical poison evolved into a biochemical necessity.
The chemistry of respiration is a chemistry of combustion. When photosynthesized hydrocarbons are jarred by the right shock, they break apart into carbon dioxide, water, and released energy—a kind of “slow” combustion. In brief, outfitted with special enzymes and antioxidants, organisms so accommodated an oxidizing atmosphere that they neutralized a potentially ruinous reaction and then absorbed and redirected it to their own ends. That, by analogy, is what terrestrial life also did when it found itself steeped in oxygen and blasted by lightning—a process of “fast” combustion we call fire.
There is more. Just as fuels exist within a larger biological context, so oxygen exists within an atmosphere. Fire responds to the air mass as a whole as well as to its selective parts. Of course without oxygen the atmosphere would be fire neutral or even a retardant. But other properties of an air mass can shape how a fire behaves. How does oxygen enter the combustion zone? Can flames expand freely upward? Will they spread in one direction rather than another? What fuels are dry? The larger properties of the air mass—its layering and stability, its winds, and its moisture (as relative humidity or storm-dropped precipitation)—will help decide. The structure of air is as vital as its chemistry, and the history of climate as relevant as the history of how the atmosphere evolved.
The question arises then whether fire has influenced the atmosphere within which it burns. Is free-burning fire a vital process in the global oxygen cycle, or simply a geochemical afterthought? Since fire and life have coevolved, have fire and the Earth's atmosphere as well? Surely combustion mediates the exchange of gases between plants and the atmosphere. It frees carbon from plants and it buries carbon as charcoal. But how much? And by regulating carbon, has fire also regulated oxygen?
Probably, but not significantly. Fire accounts for only a small fraction of global respiration. More tellingly, the linkage between oxygen's partial pressure and free-burning fire can be indirect. It is not clear that the great oxygen bubble of the later Paleozoic supported giant fires as it did giant mosquitoes. Sedimentary rocks from that era hold large stocks of charcoal, but even larger reserves of unburned biomass. Higher oxygen content does not directly translate into more fire. Spark and oxygen must still act on organic matter.
In nature, what are most important are the overall characteristics of the surrounding air and those of the fuels. The size and shape of individual particles, their chemistry, their compactness and arrangement in ways that allow oxygen to flow over their surfaces, and above all their moisture content determine whether a fire spreads from one kindled particle to another, whether combustion flames or smolders, and whether fuel burns as a surface flash or a deep-burrowing glow. Oxygen content would have to rise significantly for large, wet boles to burn, and it would have to drop hugely to prevent ignition in small, dry grasses. By whatever feedback fire shapes the atmosphere, it seems to do so through fuels. After all, the photosynthesizing plants that pump oxygen into the air are the same ones that stoke free-burning fire.
Evolving Fuels
Once stabilized, atmospheric oxygen has remained relatively uniform, much more so than ignition. Throughout the known history of fire, oxygen has persisted, a combustion constant. No surface fire has self-extinguished due to its absence. That fact has made fuels the prime biotic controller of fire. The history of combustibles, however, is nothing less than the evolution of terrestrial life.
Life had first to send its spores and extend its tendrils to land. That move exposed its photosynthesized hydrocarbons to oxygen and spark, and removed them, at least fitfully, from the smothering and cooling baths of water. Life's surge onto land injected burnable biomass into an otherwise empty combustion chamber. About the same time that vascular plants began seriously colonizing the Earth, the first evidence of fire appears in the geologic record. Yet if fire could not exist without fuel, neither would fuels—the planet's vegetative cover—exist without the evolutionary and ecological presence of fire. Each has directly shaped the other.
Follow the fuels. A field guide to fire would be a thesaurus of fuel types. Fire has acquired the vigor, subtlety, and endless variety of the organic world. The biochemistry of metabolism determines the chemistry of combustion; the ecology of biotas establishes the ecology of fire; and the evolution of new organisms shapes the evolution of fire regimes. But the reverse has also been true. Over and again fire has synthesized fuel, oxygen, and spark. Those species that could not accept this fact, like those that could not accommodate oxygen and retired to anaerobic niches, were doomed to occupy the apyric environments of the Earth.
Yet many place and periods probably did escape fire. Fire is an event, not a principle. It occurs specifically, not generically. It is easy to imagine large chunks of the Earth or blocks of Earth history that might have evaded the fusion of lightning and hydrocarbons. It is not enough that fuels bulk large on a landscape. There must be kindling—fine fuels, without which lightning blows apart rather than ignites and flame expires rather than renews. The fuels must be dry or else the heat of ignition will be wasted in boiling off the held water. They must be organized in such a way that the combustion zone can spread. Fuels—even dead fuels—are not really dead: they still flourish within a complex biological system. Their availability depends on competition with decomposers and browsers and rival species; on the climatic choreography of sun and wind, drought and rain; and on the crude timing of lightning with seasonal and secular cycles that green up and cure surface plants. It would be easy for a particular place to miss fire, and is strikingly clear from the geologic record that many did.
Locally, yes; everywhere, no. Fire demanded only certain chemical conditions, and whenever they met, it could spring into being. It could, for a while, ecologically expire, then revive. It could vanish for perhaps long eons, then return. Slowly, however, its critical parts began to interact in ways that rendered fire less random, that made its appearance and absence less like the roll of a roulette wheel and more like the give-and-take of prey and predator. It mattered not to lightning if fire happened, and most likely it mattered only marginally to oxygen. But terrestrial life would evolve with fire. Fuels were alive and could influence the character of fire in ways the pure chemistry of oxygen and the pure physics of lightning could not. By means both coarse and delicate, fire could shape the world that shaped it.
A Prehistory for Fire
Sometime between 450 and 400 million years ago, the pieces snapped together with enough force to burn and keep burning. Before that moment, fire did not exist; afterwards, it became almost impossible for it not to. The eccentric ecology of fire has since evolved along with the often lurching evolution of its parts. While the raw chemistry of combustion has remained more or less constant, fire has no more abided unchanged than has climate or life. First Fire's behavior and habitats likely looked different from today's. Triassic fires were probably as distinct as Triassic fauna and the flora they browsed and shaped. Fire's regimes during the Carboniferous, lacking grass, little resembled those typical of the Holocene, loaded with grasslands and grazers.
What were ancient fires like? Simply put, they were like the fuels on which they fed, which makes a reconstruction all the more difficult because so little is known about the range of ancient plants and how they covered the primordial Earth. The mystery is worse for the fine fuels. Small particles of combustibles—pine needles, grasses, small twigs—respond to heat and moisture more quickly than large ones do. They dry and wet faster, ignite and flare more readily. For a propagating fire, logs and peat are combustion sinks rather than sources; they may burn for a long time and release masses of carbon byproducts, but the flaming front rushes along with the small and the quick....