
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Practical Environmental Analysis
About this book
New techniques, improved understanding and changes in regulations relating to environmental analysis means that students, technicians and lecturers alike need an up-to-date guide to practical environmental analysis. This unique book provides detailed instructions for practical experiments in environmental analysis. The comprehensive coverage includes the chemical analysis of important pollutants in air, water, soil and plant tissue, and the experiments generally require only basic laboratory equipment and instrumentation. The content is supported by theoretical material explaining, amongst other concepts, the principles behind each method and the importance of various pollutants. Also included are suggestions for projects and worked examples. Appendices cover environmental standards, practical safety and laboratory practice. Building on the foundations laid by the highly acclaimed first edition, this new edition has been revised and updated to include information on new monitoring techniques, the Air Quality Index, internet resources and professional ethics. Like its predecessor, this informative text is certain to be valued as an indispensable guide to practical environmental analysis by students on a variety of science courses and their lecturers. Reviews of the first edition: "I strongly urge academics in chemistry, biology, botany, soil science, geography and environmental science departments to give [this book] serious consideration as a course text." Malcolm Cresser, Environment Department, University of York, UK "Destined to become a course text for many university courses... a high quality, informative introductory text... there should be multiple copies on most university's library shelves." Environmental Conservation
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
CHAPTER 1
Introduction
1.1 THE ENVIRONMENT
1.1.1 Biogeochemical Cycles
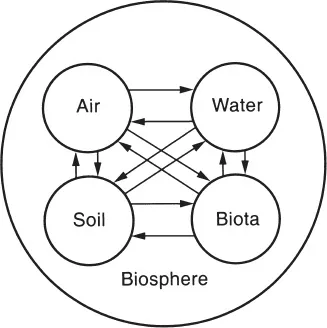

Table of contents
- Cover
- Title
- Copyright
- Preface
- Preface to the Second Edition
- Acknowledgements
- Contents
- Chapter 1 Introduction
- Chapter 2 Rainwater Analysis
- Chapter 3 Air Analysis
- Chapter 4 Water Analysis
- Chapter 5 Soil, Sediment, Sludge, and Dust Analysis
- Chapter 6 Plant Analysis
- Appendix A Safety
- Appendix B Laboratory Practice
- Appendix C Environmental Standards
- Appendix D Recommended Groundwater and Leachate Monitoring for Landfill Sites
- Appendix E Statistical Tables
- Appendix F Journals with Articles of Relevance to Environmental Analysis and Environmental Chemistry
- Appendix G Some Useful Websites
- Subject Index
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app