
Advances in Biological Solid-State NMR
Proteins and Membrane-Active Peptides
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Advances in Biological Solid-State NMR
Proteins and Membrane-Active Peptides
About this book
The complexity and heterogeneity of biological systems has posed an immense challenge in recent years. An increasingly important tool for obtaining molecular and atomic scale information on a range of large biological molecules and cellular components is solid-state NMR. This technique can address fascinating problems in structural biology, including the arrangement of supramolecular complexes and fibril formation in relation to molecular folding, misfolding and aggregation.
Advances in Biological Solid-State NMR brings the reader up to date with chapters from international leaders of this growing field, covering the most recent developments in the methodology and applications of solid-state NMR to studies of membrane interactions and molecular motions. A much needed discussion of membrane systems is detailed alongside important developments in in situ analysis.
Topics include applications to biological membranes, membrane active peptides, membrane proteins, protein assemblies and in-cell NMR. This exposition of an invaluable technique will interest those working in a range of related spectroscopic and biological fields. A basic introduction invites those interested to familiarise themselves with the basic mathematical and conceptual foundations of solid-state NMR. A thorough and comprehensive discussion of this promising technique follows, which is essential reading for those working or studying at postgraduate level in this exciting field.
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
CHAPTER 1
Introduction to Biological Solid-State NMR
1.1 Preface
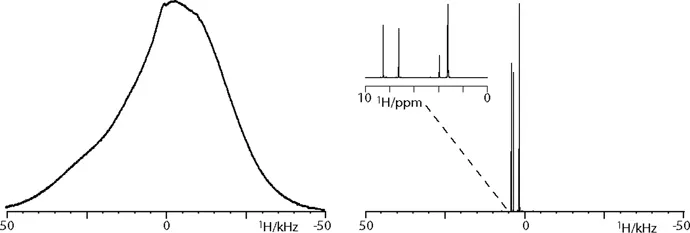
1.2 Interactions in Biological Solid-State NMR
1.2.1 General and Rotational Properties of NMR Interactions
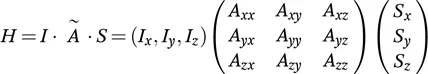
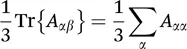
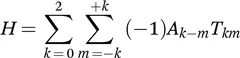
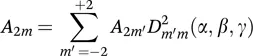
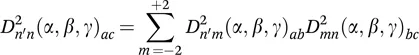
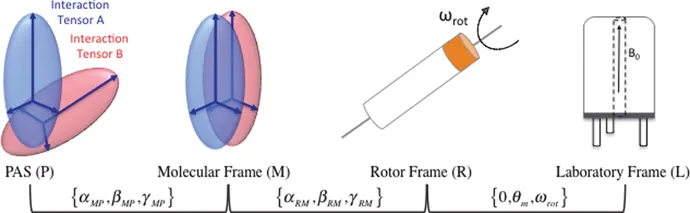
1.2.2 Chemical Shift Anisotropy
Table of contents
- Cover
- Title
- Copyright
- Contents
- Chapter 1 Introduction to Biological Solid-State NMR
- Chapter 2 Combining NMR Spectroscopic Measurements and Molecular Dynamics Simulations to Determine the Orientation of Amphipathic Peptides in Lipid Bilayers
- Chapter 3 13C–13C Distance Measurements by Polarization Transfer Matrix Analysis of 13C Spin Diffusion in a Uniformly 13C-Labeled Molecular Complex under Magic Angle Spinning
- Chapter 4 Demonstration of the Equivalence of Solid-State NMR Orientational Constraints from Magnetic and Rotational Alignment of the Coat Protein in a Filamentous Bacteriophage
- Chapter 5 Membrane Protein Interactions
- Chapter 6 Magnetic Liposomes and Bicelles: New Tools for Membrane-Peptide Structural Studies
- Chapter 7 Membranes and Their Lipids: A Molecular Insight into Their Organization and Function
- Chapter 8 Structural Studies of Small Bioactive Compounds Interacting with Membranes and Proteins
- Chapter 9 Lipopolysaccharide Induces Raft Domain Expansion in a Cholesterol-Containing Membrane
- Chapter 10 Deuterium NMR of Mixed Lipid Membranes
- Chapter 11 Membrane Interactions of Amphiphilic Peptides with Antimicrobial Potential: A Solid-State NMR Study
- Chapter 12 Investigations of the Structure, Topology and Dynamics of Membrane-Associated Polypeptides by Solid-State NMR Spectroscopy
- Chapter 13 NMR of Lipids and Lipid/Peptide Mixtures
- Chapter 14 NMR Investigations of the Structure and Dynamics of Antimicrobial Peptides: The Peptaibol Alamethicin
- Chapter 15 Solid-State NMR Studies of Antimicrobial Peptide Interactions with Specific Lipid Environments
- Chapter 16 Dynamic Structure Analysis of Peptides in Membranes by Solid-State NMR
- Chapter 17 Structural Dynamics of Retinal in Rhodopsin Activation Viewed by Solid-State 2H NMR Spectroscopy
- Chapter 18 Helical Membrane Protein Structure: Strategy for Success
- Chapter 19 Chemistry and Structure via Solid-State NMR
- Chapter 20 Photoactivated Structural Changes in Photoreceptor Membrane Proteins as Revealed by in situ Photoirradiation Solid-State NMR Spectroscopy
- Chapter 21 A Promising Prognosis for Solid-State NMR of Functional Membrane Protein Complexes
- Chapter 22 Structural Topologies of Phosphorylated and Non-phosphorylated Oligomeric Phospholamban in Lipid Membranes by a Hybrid NMR Approach
- Chapter 23 Structural Insights from Solid-State NMR into the Function of the Bacteriorhodopsin Photoreceptor Protein
- Chapter 24 2H Solid-State NMR Study of Peptide–Membrane Interactions in Intact Bacteria
- Chapter 25 Magic Angle Spinning NMR Spectroscopy for Resolving Structure and Mechanisms of Function of Membrane Protein Assemblies Involved in Photosynthetic Energy Conversion
- Chapter 26 Large Protein Complexes Revealed by Solution-State NMR: G Proteins and G Protein-Activated Inwardly Rectifying Potassium Ion Channel
- Chapter 27 NMR Studies of Small Molecules Interacting with Amyloidogenic Proteins
- Chapter 28 Solid-State NMR Studies of β-Amyloid Fibrils and Related Assemblies
- Subject Index