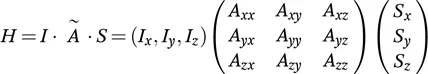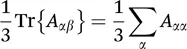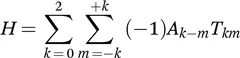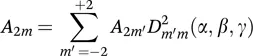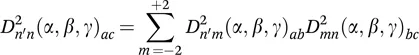M. WEINGARTH AND M. BALDUS*
1.1 Preface
Solid-state nuclear magnetic resonance has emerged as an established spectroscopic technique to provide atomic-scale information in complex biological systems. Recent years, with the appearance of the first de novo structures of membrane proteins1 and solid-state NMR spectroscopists unraveling the details of native cellular components at atomic resolution,2 indeed seem to mark a watershed moment for solid-state NMR becoming a leading technique to study highly disordered or heterogeneous biological systems.
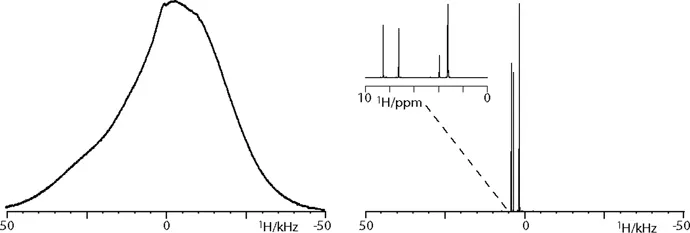
These stunning advances, however, are hardwon. What catches one’s eye, especially for eyes used to the spectral resolution of liquid-state NMR, is the broadness of non-modulated solid-state NMR signals. For liquid-state spectroscopists, such data may appear as a featureless blob, from which structural parameters are hardly deducible, let alone at atomic resolution (Figure 1.1). The broadness of the signals, which easily exceeds dozens of kHz or even several MHz, results from the presence of anisotropic interactions in solid-state NMR spectra, and a large part of solid-state NMR methodology refers to the manipulation of the Hamiltonian to dissect the anisotropic interactions or to suppress their influence on NMR spectra in a controlled manner.
Figure 1.1 Comparison of a conventional proton spectrum of natural abundance histidine measured in solution (right) and as a static solid powder (left). While anisotropic interactions broaden the lines in solid samples, nature solves this issue in liquid samples by rapid molecular tumbling, which is usually orders of magnitude larger than the anisotropic interactions.
In the following, we will first introduce the reader to the most relevant NMR interactions in solid-like biological systems and afterwards briefly discuss the basic components of solid-state NMR pulse sequences for biological applications.
1.2 Interactions in Biological Solid-State NMR
Interactions which affect the spin system and its associated Hamiltonian can be classified as external and internal (eqn 1.1). The external Hamiltonian is directly under the control of the NMR operator and consists of the Zeeman interaction and the rf (radio-frequency) fields, while the internal Hamiltonian includes the interaction of the spins with the local electronic environment (chemical shift), with each other (dipolar and scalar couplings) and with electric field gradients (quadrupolar coupling). The internal interactions may be modulated by the spectroscopist by means of rf pulses or magic angle spinning.
Htotal=Hexternal+Hinternal (1.1)
1.2.1 General and Rotational Properties of NMR Interactions
Before we summarize the most relevant interactions in biological solid-state NMR, we will briefly discuss their general appearance and properties. Whether external or internal interactions, their Hamiltonians can be depicted as:
with I and S as row and column vectors, respectively, and à as a second-rank Cartesian tensor as a means to represent the anisotropy of an interaction, corresponding to a 3×3 square matrix. In this equation, I is a spin operator, while the other vector S represents either a second spin (dipolar or scalar coupling), or a magnetic (chemical shift) or an electric field (quadrupolar coupling). Subjected to rapid isotropic molecular motion, à is averaged to its trace, which is the sum of the diagonal elements:
Besides the Cartesian representation, irreducible spherical tensor operators are a particularly appropriate basis to describe transformations in solid-state NMR:
In this representation, it is easily visible that any NMR interaction can be broken down into a spatial part, represented by spherical tensor A, and a spin part, represented by spherical tensor operators T. Indices k indicate the rank of a tensor, which has 2m+1 orders (which are irreducible spherical tensors). Component A00 is the isotropic, A1−m is the antisymmetric and A2−m is the anisotropic part of an interaction. Since the antisymmetric part can usually be ignored, and since the static magnetic field B0 is usually orders of magnitude larger than the internal interactions (with the exception of certain quadrupolar interactions), the summation can be reduced in the so-called secular approximation to:
H=A00T00+A20T20 (1.5)
neglecting all terms that vanish over time and retaining only those that commute with B0. Rotations of spherical tensors are carried out by Wigner rotation matrices D of the same rank, with the Euler angles α, β and γ (eqn 1.6). Details of all these operations can be found in standard references.3
NMR interactions have their simplest form in their principal axis system (PAS), in which all off-diagonal elements are zero. Yet, NMR signals are detected in the laboratory frame, into which the interactions thus have to be transformed to analyze their effect on the spectrum. Transformations between different frames obey the following relation, which is called the addition theorem of Wigner rotation matrices:
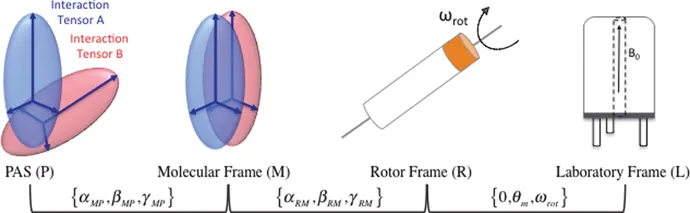
According to these rules, NMR interactions can be transformed from one frame into another. Typically, in solid-state NMR, transformations include a transformation from the PAS to a molecular frame, which is fixed to the molecular structure and in which all NMR interactions share a common orientation, followed by a transformation to a rotor frame to take into account the powder averaging, and finally into the laboratory frame (Figure 1.2).
Figure 1.2 Pictorial representation of the usual transformations in solid-state NMR.
1.2.2 Chemical Shift Anisotropy
Nuclei possessing a spin I have an associated nuclear magnetic dipole moment μ=γħI, with γ as the gyromagnetic ratio. For an isolated spin-½ nucleus, subjected to a ...