
Paramagnetism in Experimental Biomolecular NMR
- 316 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Paramagnetism in Experimental Biomolecular NMR
About this book
Paramagnetic NMR is a growing technique that represents an increasingly important tool for the investigation of biomolecules. This book presents an update and overview of the paramagnetic NMR phenomena and effects as well as guidelines for practical implementation of state-of-the-art experiments. All experiments are supported by a solid theoretical foundation. Areas mentioned are the development of solid state NMR, the use of paramagnetic tags providing information on the structure and mobility of the investigated systems, and dynamic nuclear polarization to increase sensitivity.
Compiled by experts in the field, this book has international appeal for researchers as well as students interested in magnetic resonance and structural biology who require experimental support and accessible information.
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
*Email: [email protected]
1.1 The Effect of Paramagnetism on NMR Spectra
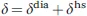
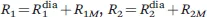
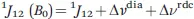
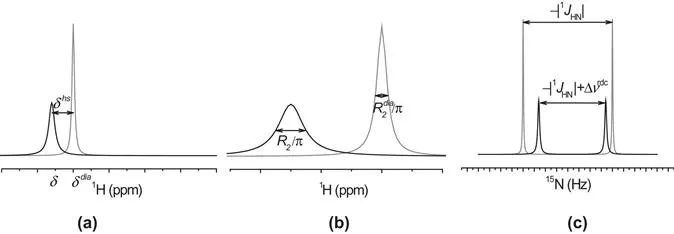
1.1.1 The Hyperfine Coupling
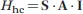
Table of contents
- Cover
- Title
- Copyright Page
- Preface
- List of Acronyms
- List of Symbols
- Contents
- Chapter 1 NMR Consequences of the Nucleus–Electron Spin Interactions
- Chapter 2 Intrinsic and Extrinsic Paramagnetic Probes
- Chapter 3 Structural and Dynamic Characterization of Protein Domains using Paramagnetic Data
- Chapter 4 Treating Biomacromolecular Conformational Variability
- Chapter 5 Protein–Protein Interactions
- Chapter 6 Solid-state NMR of Paramagnetic Proteins
- Chapter 7 Relaxometry and Contrast Agents
- Chapter 8 Dynamic Nuclear Polarization
- Chapter 9 Paramagnetic NMR in Drug Discovery
- Chapter 10 Small Paramagnetic Co-solute Molecules
- Subject Index