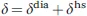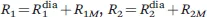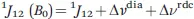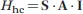![]()
CHAPTER 1
NMR Consequences of the Nucleus–Electron Spin Interactions
GIACOMO PARIGIa,b AND CLAUDIO LUCHINAT*a,b
a Magnetic Resonance Center (CERM), via L. Sacconi 6, Sesto Fiorentino 50019, Italy;
b Department of Chemistry “Ugo Schiff”, University of Florence, via della Lastruccia 3, Sesto Fiorentino 50019, Italy
*Email:
[email protected] 1.1 The Effect of Paramagnetism on NMR Spectra
The presence of unpaired electrons in molecules makes them paramagnetic and largely affects their NMR spectra. The main effects related to the presence of a paramagnetic center, i.e., of atoms or ions with unpaired electrons, are (see Figure 1.1):1
(1) the NMR shifts are perturbed, so that the shifts measured for the paramagnetic molecule (δ) and for a diamagnetic analogue (δdia) (i.e., for the same molecule without the paramagnetic center or with the paramagnetic metal ion substituted by a diamagnetic one) differ. These differences are called hyperfine shifts (δhs):
(2) the nuclear relaxation rates of the paramagnetic molecule (R1, longitudinal; R2, transverse) are increased with respect to those of a diamagnetic analogue (Rdia1 and Rdia2). This difference is called paramagnetic relaxation enhancement (R1M and R2M):
(3) the probabilities for the different orientations of the paramagnetic molecule in a magnetic field are not the same, so a partial self-orientation occurs. This partial self-orientation is responsible for the occurrence of residual dipolar couplings (Δνrdc), analogously to what happens when partial molecular orientation is driven by external devices. The presence of paramagnetic residual dipolar couplings affects the J-coupling between nuclei:
This chapter deals with the description of these effects, and of their physical origin, which is related to the so-called hyperfine coupling. In eqn (1.1)–(1.3) other minor effects, namely residual anisotropic chemical shifts, cross relaxation terms, and dynamic frequency shifts, respectively, have been neglected (see later).
Figure 1.1 The presence of a paramagnetic center affects NMR shifts (a), relaxation rates (b) and 1J splitting (c).
1.1.1 The Hyperfine Coupling
The interaction between the magnetic moment associated to a nuclear spin I and the magnetic moment associated to an unpaired electron spin S is called hyperfine coupling and is traditionally described by the Hamiltonian
The energy corresponding to this interaction fluctuates with time (and thus this interaction represents a source of relaxation for the nuclear spin, see Section 1.4) as a result of the molecular motions and of changes in the electron spin state. The other terms describing the contributions to the energy of the system are:
(i) the Zeeman interaction between the applied magnetic field B0 and the nuclear magnetic moment μI=ħγII, where ħ is Planck's constant divided by 2π and γI is the nuclear magnetogyric ratio,
(ii) the Zeeman interaction between B0 and an ‘effective’ electron magnetic moment taking into account both the electron spin and the orbital magnetic moments. This is parameterised using the g tensor, with the effective electron magnetic moment given by μS=−μBg · S, where μB is the electron Bohr magneton. Therefore, the g tensor couples the ‘effective’ electron spin to B0, thus accounting for the presence of the anisotropic orbital contributions to the electron ma...