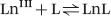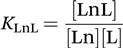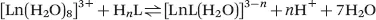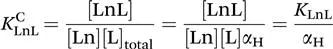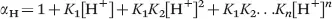QUYEN N. DO, JAMES S. RATNAKAR AND ZOLTÁN KOVÁCS*
1.1.1 Relationships between Ligand Structure and Complex Properties
GdIII-based contrast agents for MRI are used in approximately 30% of MRI exams.1 Although the FDA-approved contrast agents are among the safest drugs on the market, their core, the GdIII ion, has a 50% lethal dose, (LD50) around 0.1–0.2 mmol kg−1.2 Therefore, for medical diagnostic applications, GdIII ions must be chelated by ligands to prevent the metal ion from being released. Administration of complexes with insufficient kinetic inertness can result in the debilitating disease nephrogenic systemic fibrosis (NSF), deposition of metal in the brain, or other issues.3–5 To develop safe and efficient GdIII-based (Chapter 2) and lanthanide-based CEST agents (Chapter 3) for MRI, it is important to understand the role of the ligand in determining the relaxivity, thermodynamic stability, and kinetic inertness of complexes. The fundamentals described in this chapter are intended to assist with the design of future imaging agents for MRI.
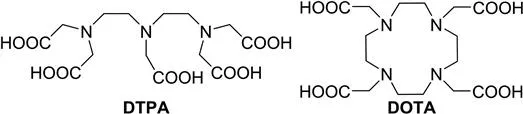
Ligands play a critical role not only in reducing the toxicity of metal ions, but also in optimizing the parameters that determine the relaxivity or CEST-enhancing ability of metal complexes. These parameters include the number of inner-sphere water molecules (q), the residence lifetime of coordinated inner-sphere water molecules (τM), and the rotational correlation time (τR). Chelators sometimes contain moieties for biosensing or reactive functionalities as points of attachment for targeting vectors. All chelators currently used in clinically approved contrast agents for MRI are octadentate ligands based either on the open-chain ligand diethylenetriaminepentaacetic acid (DTPA) or the macrocyclic ligand 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) (Figure 1.1). Most reported responsive and bifunctional ligands are also derivatives of these two chelators. With both of these ligands, the ninth coordination site of GdIII is occupied by a rapidly exchanging water molecule that transfers the paramagnetic relaxation effect of the metal ion to the pool of bulk water. Properties of ligands, such as total basicity (sum of the protonation constants), basicity of the first protonation site, preorganization, and rigidity, influence the thermodynamic stability and kinetic behavior of the resulting metal complexes.
Figure 1.1 The two standard ligand scaffolds of clinically used GdIII complexes: DTPA and DOTA.
The bonding in lanthanide complexes is predominantly ionic, and coordination geometries are largely determined by the steric bulk of the ligands. Because LnIII ions are considered to be hard Lewis acids, they favor hard donor atoms, such as fluoride, oxygen, and, to a lesser extent, nitrogen. LnIII ions typically have coordination numbers of eight or nine, and they can form stable complexes with ligands that have matching denticities. LnIII ions form relatively stable complexes with ligands that enable the formation of five-membered chelate rings; ligands that form six-membered chelate rings are far less stable.6–12
The thermodynamic stability of a complex is expressed as the equilibrium constant written for the reaction between the free metal ion and the fully deprotonated ligand [eqn (1.1)].13–15
The stability constant of the complex, KLnL, is defined as follows:
In aqueous solution, the ligand can be fully or partially protonated depending on both the pH of the solution and the protonation constants (basicity) of the ligand. The formation of the metal complex is thus essentially a competition between protons and the metal ion for the donor sites of the ligand. Consequently, the true stability of a complex at a given pH is determined by its conditional stability constant, KCLnL, which takes into account the protonation of the ligand. Thus, for the formation of a lanthanide chelate with one inner-sphere water molecule, the stability of the complex is characterized by the following equilibrium.
In eqn (1.4), [L]total is the total concentration of the free and protonated ligand species that are not bound to the lanthanide ion, and αH is the total or equilibrium ligand concentration ratio.14–17αH is expressed using the [H+] and the protonation constants of the ligand as:
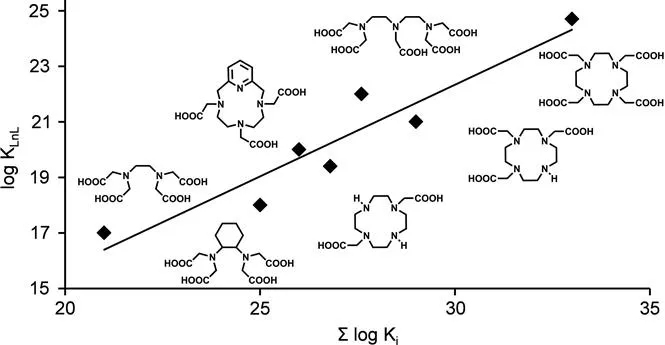
For polyaminopolycarboxylate ligands, there is a nearly linear relationship between the basicity of the ligand, determined in terms of the sum of the protonation constants, Σlog Ki, and the stability of the corresponding GdIII complexes (Figure 1.2). Deviations were reported for ligands that do not form five-membered chelate rings, for ligands that have non-coordinating peripheral groups that undergo protonation, and for non-polyaminocarboxylate ligands. For DTPA- and DOTA-type ligands, more basic ligands tend to form complexes with higher stabilities.15,16
Figure 1.2 Linear correlation between the stability of the GdIII complexes and the total basicity of the ligands for common polyaminopolycarboxylates. The graph is based on stability constant data reported in ref. 16.
According to the chelate effect, metal complexes with polydentate ligands are more stable than similar complexes with the same number of monodentate ligands. Macrocyclic ligands with the same number of donor atoms as linear ligands tend to be even more stable; this is known as the macrocyclic effect.6,18–20 The chelate effect is driven by entropy because the number of particles increases as l...