
Analytical Characterization of Biotherapeutics
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Analytical Characterization of Biotherapeutics
About this book
The definitive guide to the myriad analytical techniques available to scientists involved in biotherapeutics research
Analytical Characterization of Biotherapeutics covers all current and emerging analytical tools and techniques used for the characterization of therapeutic proteins and antigen reagents. From basic recombinant antigen and antibody characterization, to complex analyses for increasingly complex molecular designs, the book explores the history of the analysis techniques and offers valuable insights into the most important emerging analytical solutions. In addition, it frames critical questions warranting attention in the design and delivery of a therapeutic protein, exposes analytical challenges that may occur when characterizing these molecules, and presents a number of tested solutions.
The first single-volume guide of its kind, Analytical Characterization of Biotherapeutics brings together contributions from scientists at the leading edge of biotherapeutics research and manufacturing. Key topics covered in-depth include the structural characterization of recombinant proteins and antibodies, antibody de novo sequencing, characterization of antibody drug conjugates, characterization of bi-specific or other hybrid molecules, characterization of manufacturing host-cell contaminant proteins, analytical tools for biologics molecular assessment, and more.
- Each chapter is written by a recognized expert or experts in their field who discuss current and cutting edge approaches to fully characterizing biotherapeutic proteins and antigen reagents
- Covers the full range of characterization strategies for large molecule based therapeutics
- Provides an up-to-date account of the latest approaches used for large molecule characterization
- Chapters cover the background needed to understand the challenges at hand, solutions to characterize these large molecules, and a summary of emerging options for analytical characterization
Analytical Characterization of Biotherapeutics is an up-to-date resource for analytical scientists, biologists, and mass spectrometrists involved in the analysis of biomolecules, as well as scientists employed in the pharmaceuticals and biotechnology industries. Graduate students in biology and analytical science, and their instructors will find it to be fascinating and instructive supplementary reading.
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
1
Introduction to Biotherapeutics
Abbreviations
- ADAs
- antidrug antibodies
- ADC
- antibody–drug conjugate
- ADCC
- antibody‐dependent cell‐mediated cytotoxicity
- CDR
- complementary‐determining region
- Fab
- antigen binding fragment
- Fc
- cystallizable fragment
- NMR
- nuclear magnetic resonance
- PEG
- polyethyleneglycol
- PTM
- posttranslational modification
1.1 Introduction
1.2 Types of Biotherapeutics and Manufacturing Systems

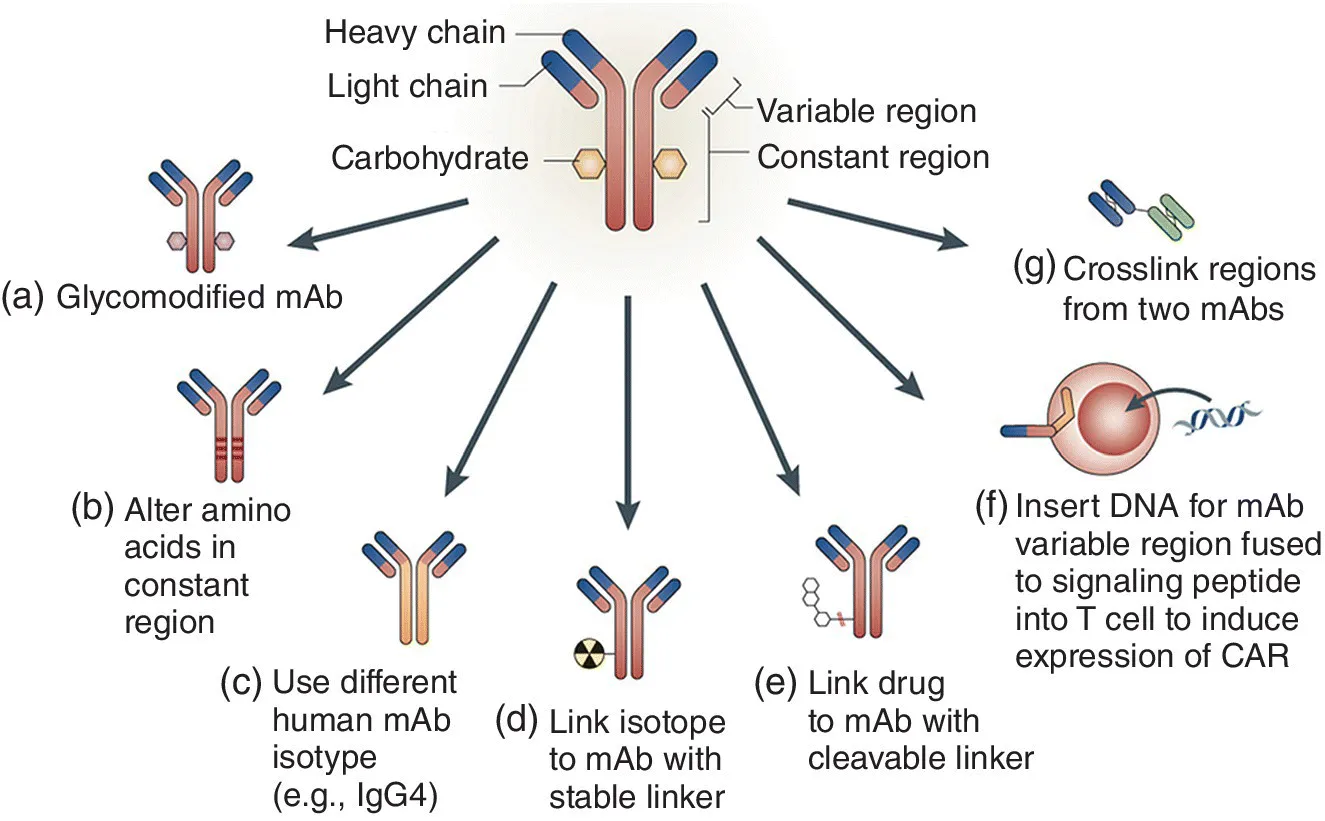
Table of contents
- Cover
- Title Page
- Table of Contents
- List of Contributors
- 1 Introduction to Biotherapeutics
- 2 Mass Spectrometric Characterization of Recombinant Proteins
- 3 Characterizing the Termini of Recombinant Proteins
- 4 Assessing Activity and Conformation of Recombinant Proteins
- 5 Structural Characterization of Recombinant Proteins and Antibodies
- 6 Antibody de novo Sequencing
- 7 Characterization of Antibody–Drug Conjugates
- 8 Characterization of Bispecific or Other Hybrid Molecules
- 9 Bio‐Repository
- 10 Characterization of Residual Host Cell Protein Impurities in Biotherapeutics
- 11 Analytical Tools for Biologics Molecular Assessment
- 12 Glycan Characterization
- Index
- End User License Agreement